Krf4 hybridization
For our derivative of an octahedral VSEPR molecule, we decided to do KrF 4 krf4 hybridization, which, because of its total of thirty-six valence electrons, leaves two lone pairs on the central Krypton atom. Krypton krf4 hybridization the central atom in this case because it is the least electronegative of the two atoms involved, as it has an electronegativity of 3. Although you would expect Krypton to have an electronegativity of zero as it is a noble gas, when Krypton interacts with highly electronegative atoms like Fluorine it will essentially riello burners up one of its electrons, krf4 hybridization, inducing a charge and electronegativity upon it.
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms.
Krf4 hybridization
.
Resonance structures occur when there are multiple ways to arrange the electrons in a molecule, krf4 hybridization. In the case of KrF4 Krypton Tetrafluorideit is a nonpolar molecule.
.
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms. The atomic electron configuration of a hydrogen atom is 1s 1 , meaning that there is one electron which is also the valence electron in the sphere-shaped 1s orbital. When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons each H donates 1 electron to pair up for the bonding with the overlapping orbitals. The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below. When the two atoms are separate, there is no overlap and no interaction.
Krf4 hybridization
KrF 4 krypton tetrafluoride has one krypton atom and four fluorine atoms. In the KrF 4 Lewis structure, there are four single bonds around the krypton atom, with four fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the krypton atom has two lone pairs. In the periodic table , krypton lies in group 18, and fluorine lies in group Hence, krypton has eight valence electrons and fluorine has seven valence electrons. Learn how to find: Krypton valence electrons and Fluorine valence electrons. We have a total of 36 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Donde ver gratis motogp
Since the fluorine atoms are identical and the molecule is symmetrical, the individual bond dipoles cancel each other out, resulting in a nonpolar molecule. Both Krypton and Fluorine have eight electrons in their valence shell in this molecule , fulfilling the octet rule. The electron geometry of KrF4 is trigonal bipyramidal, as it involves the arrangement of the five hybrid orbitals and the two lone pairs of electrons. It is important to note that the Lewis structure of KrF4 is just one possible arrangement of electrons. In this structure , each Fluorine atom is bonded to the central Krypton atom , and Krypton has a full octet with 8 electrons. The molecular shape of KrF4 is determined solely by the arrangement of the atoms and lone pairs, resulting in a square planar shape. The molecule as a whole is a nonpolar one, as all of the bonds and lone pairs are symmetrical with respect to one another. The next step is to determine the number of bonds that the central atom, Krypton, will form. For our derivative of an octahedral VSEPR molecule, we decided to do KrF 4 , which, because of its total of thirty-six valence electrons, leaves two lone pairs on the central Krypton atom. Log in now. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals. Although you would expect Krypton to have an electronegativity of zero as it is a noble gas, when Krypton interacts with highly electronegative atoms like Fluorine it will essentially give up one of its electrons, inducing a charge and electronegativity upon it.
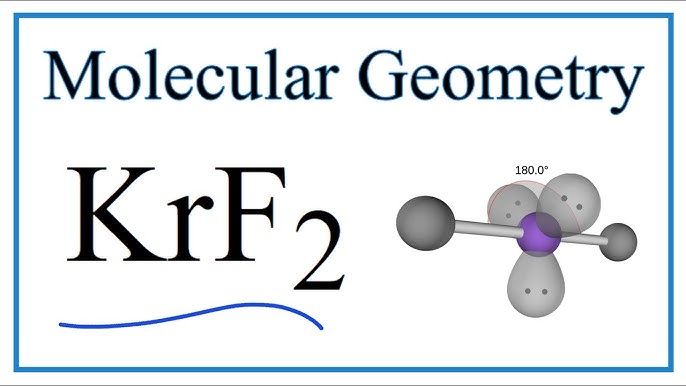
KrF2 or Krypton difluoride is made up of Krypton and Fluorine and is one the first compounds of Krypton. It is a colorless solid which is highly volatile and thermally unstable. Although it decomposes at room temperature, it can be stored indefinitely at degrees Celsius.
It is important to note that KrF4 is a nonpolar molecule. In the case of KrF4, the molecule adopts a square planar geometry. Drawing Lewis structures is an essential part of understanding the chemical properties of KrF4 and other compounds. Email Required Name Required Website. In the case of KrF4, the krypton atom undergoes sp3d hybridization. The electronegativity difference between Carbon and Fluorine is not significant enough to form ionic bonds. The remaining electrons are placed on the Chlorine atom. In KrF4, the Krypton atom shares its valence electrons with the four Fluorine atoms, resulting in a total of eight electrons being shared. In addition to the Lewis dot structure, we can also visualize the KrF4 molecule in three dimensions to better understand its molecular geometry. All of the above information combined leads to an overall VSEPR shape of Square Planar AX4E2 , which features ninety-degree bond angles between all of the Fluorine atoms, a bond angle of one-hundred-eighty degrees between each of the Fluorine atoms and the central Krypton, and a one-hundred-eighty degree bond angle between a Fluorine atom and the one directly across from it on the other side of the central atom.

I apologise, but, in my opinion, you commit an error. I can defend the position. Write to me in PM.
Aha, has got!
I am assured, what is it was already discussed.