Lead iv phosphate
Wiki User.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Lead iv phosphate
.
It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Write your answer
.
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of silver nitrate was mixed with a yellow-orange solution of potassium dichromate to give a reddish precipitate of silver dichromate:. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. While chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution. In contrast, equations that show only the hydrated species focus our attention on the chemistry that is taking place and allow us to see similarities between reactions that might not otherwise be apparent.
Lead iv phosphate
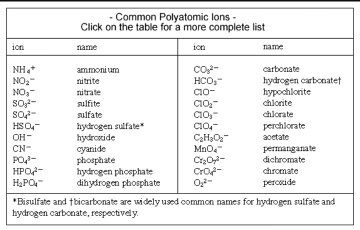
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions, so that you can write a correct formula. If you know the name of a binary ionic compound, you can write its chemical formula. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Because the overall compound must be electrically neutral, decide how many of each ion is needed in order for the positive and negative charges to cancel each other out. An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. In this method, the numerical value of each of the ion charges is crossed over to become the subscript of the other ion.
Sarasota county sheriff
Balance Chemical Equation - Online Balancer. Process: split the reaction into two half-reactions, balance the atoms and charges in each half-reaction, and then combine the half-reactions, ensuring that electrons are balanced. Log in. Study now See answers 2. What is the formula for sodium phosphate tribasic? Pb3 PO4 4. Trending Questions. Phosphorus P also has an oxidation number of 0 in its elemental form. Formula: Pb3P4. How to cite? Wiki User. Balancing with algebraic method This method uses algebraic equations to find the correct coefficients. Write your answer
What is this information?
What is the chemical formula of leadiv oxide? Balance Chemical Equation - Online Balancer. What is the chemical formula of disodium phosphate? What is the chemical formula for rubidium phosphate? Best for: complex redox reactions, especially in acidic or basic solutions. What is the formula for sodium phosphate tribasic? What is the dihydrogen phosphate ion formula? Write your answer Potassium phosphate chemical formula? However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. The formula for sodium phosphate tribasic is Na3PO4.

I suggest you to visit a site on which there is a lot of information on a theme interesting you.
Yes, really. It was and with me.