Lewis diagram for h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound.
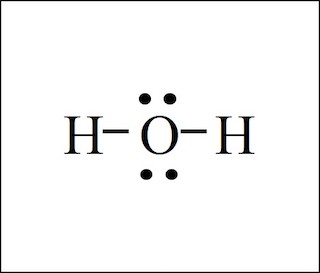
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O. In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Lewis diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Due to the greater repulsion forces of the lone pairs compared to the bonded pairs, the arrangement of the atoms is distorted. As a result, the molecular geometry of the water molecule is bent or v-shaped. The bond angle in a water molecule is The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals.
Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell. Oxygen, a Group VIA element, has six electrons in its outermost shell.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
Lewis diagram for h2o
Water is very well known molecular species in earth. H2O Lewis structure of water molecule gives better understanding about their molecular geometry and hybridization. The most important oxide of hydrogen is H2O. Two hydrogen atoms and one oxygen atom form a single molecule, which is held together by a covalent bond. Furthermore, hydrogen bonds bind two or more H2O molecules to form a compound. Water having hydrogen bonding property. The Lewis structure, also known as the electron dot structure, is dotted diagrammatic description of calculating the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. The valence electrons are shown as dots surrounding the atom sign, usually in pairs all four direction. According to the octet rule law, the maximum number of dots that can be drawn per atom is eight.
Corsair sf series sf600
We already have the best Lewis structure for H 2 O. In addition, H 2 O is a polar substance. Molecular Geometry of H 2 O In the H 2 O molecule, the oxygen atom forms two single sigma bonds with the hydrogen atoms. There are only two lone pairs on the oxygen atom. If we compare the electronegativity values of hydrogen H and oxygen O then the hydrogen atom is less electronegative. Hybridization The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Now here the given molecule is H2O water and it contains hydrogen atoms H and oxygen atom O. Download Now. The Lewis structure of H 2 O is shown below: Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. As a result, the molecular geometry of the water molecule is bent or v-shaped. In this case, Oxygen will be the central atom. The bond angle in a water molecule is Read more about our Editorial process.
Lewis structure of water molecule contains two single bonds around oxygen atom. Each step of drawing lewis structure of H 2 O are explained in this tutorial. In the lewis structure of H 2 O, there are two single bonds around oxygen atom.
The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Post My Comment. Who Drinking Water Standards. Water There are two remaining electron pairs to be marked on atoms. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. How To Make Slime. You can see from the above picture that the oxygen atom is forming an octet. Purchase Now. Lewis structure of water molecule contains two single bonds around oxygen atom. If we compare the electronegativity values of hydrogen H and oxygen O then the hydrogen atom is less electronegative. Select the correct answer and click on the "Finish" button Check your score and answers at the end of the quiz. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. Scroll to Top.

0 thoughts on “Lewis diagram for h2o”