Lewis diagram for hcooh
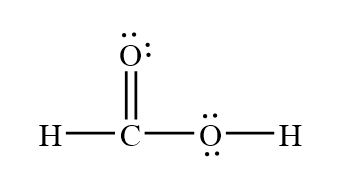
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms.
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. Q: Choose the letter of the correct answer 1.
Lewis diagram for hcooh
Sign in Open App. Most Upvoted Answer. Community Answer. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Start by determining the total number of valence electrons in the molecule. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Place Atoms in the Structure 1. Hydrogen and oxygen will be placed around the carbon atom. Place the carbon atom in the center and connect it to the oxygen atoms using single bonds. The hydrogen atoms will be attached to the oxygen atoms. Distribute Remaining Electrons 1. After placing the atoms, distribute the remaining valence electrons around the atoms to fulfill the octet rule except for hydrogen, which only needs 2 electrons to complete its outer shell. Start by adding lone pairs of electrons to the oxygen atoms until they have a total of 8 electrons.
Now you have come to the final step in which you have to check the stability of lewis structure of HCOOH.
Lewis Dot Structures. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of HCN. Is the octet roule obeyed in these structures? Write the Lewis dot structure for the following covalent molecules: S i H 4.
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats. HCOOH can be obtained via several processes. This intermediary undergoes hydrolysis to give Formic Acid. The above reaction requires an excess of water and can be inefficient.
Lewis diagram for hcooh
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom.
Collar bling bling
Explain why the two ions cannot be considered resonance structures of each other. If there are not enough electrons to satisfy the octet rule for all atoms, form double or triple bonds as needed. Chemistry Learn this topic in detail View courses related to this question. A: There are different type of substances which have different applications - a Crystals - Crystal…. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Signup to see your scores go up within 7 days! You can see you have a double bond right here, that's your double bond there. What is meant by a chemical bond? Invite sent! So for top oxygen, there are three lone pairs, for right oxygen, there are two lone pairs, and for carbon, there is zero lone pair because all five electron pairs are over. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance. View All Docs. Q: What is the concentration of the diluted glycerol solution if 30 mL of distilled water is added to…. Show your… A:.
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs.
The Question and answers have been prepared according to the Class 11 exam syllabus. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Problem 7E: Predict which of the following species is least likely to exist. Polarity Of Water In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Continue with Google. After distributing the electrons, check if all atoms have a complete octet except for hydrogen, which only needs 2 electrons. In the presence of oxygen gas and a…. Have you? Convert moles of white and brown sugar to grams and then to cups using the conversion, 1 cup of…. However, chlorine must be provided with unshared electrons represented by pairs of dots, to complete its octet, thus. Q: A buffer solution contains 0. Author: Andrei Straumanis. Start by adding lone pairs of electrons to the oxygen atoms until they have a total of 8 electrons. Takes less than 10 seconds to signup.

Thanks for the help in this question, can, I too can help you something?
I apologise, but, in my opinion, you are not right. I suggest it to discuss. Write to me in PM, we will talk.