Lewis dot diagram for c2h6
Ethane is an organic compound with a chemical formula of C2H6, lewis dot diagram for c2h6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures.
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer. Open in App. Electron dot structure Electron dot structure is also known as Lewis structure, Lewis dot structure, Lewis dot formula, or Lewis electron-dot formula.
Lewis dot diagram for c2h6
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is C2H6 or ethane and it contains carbon atoms C and hydrogen atoms H. You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. If we compare the electronegativity values of carbon C and hydrogen H then the hydrogen atom is less electronegative.
Q: Maybelline Cousteau's backup oxygen tank reads mmHg while on her boat, where the temperature is…. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane.
The covalent bonding in the ethane molecule. All my GCSE level chemistry revision notes. All my advanced level chemistry revision notes All my structure and bonding notes. Carbon's electrons are 2. Two electronic diagrams for ethane C 2 H 6. Venn diagram style of showing the bonds in ethane. I hope you like the atomic colour coding - xref physics!
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1. All of these are sigma bonds. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl CH 3 groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other. The sp 3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. Just like the carbon atom in methane, the central nitrogen in ammonia is sp 3 -hybridized. With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a non-bonding pair of electrons.
Lewis dot diagram for c2h6
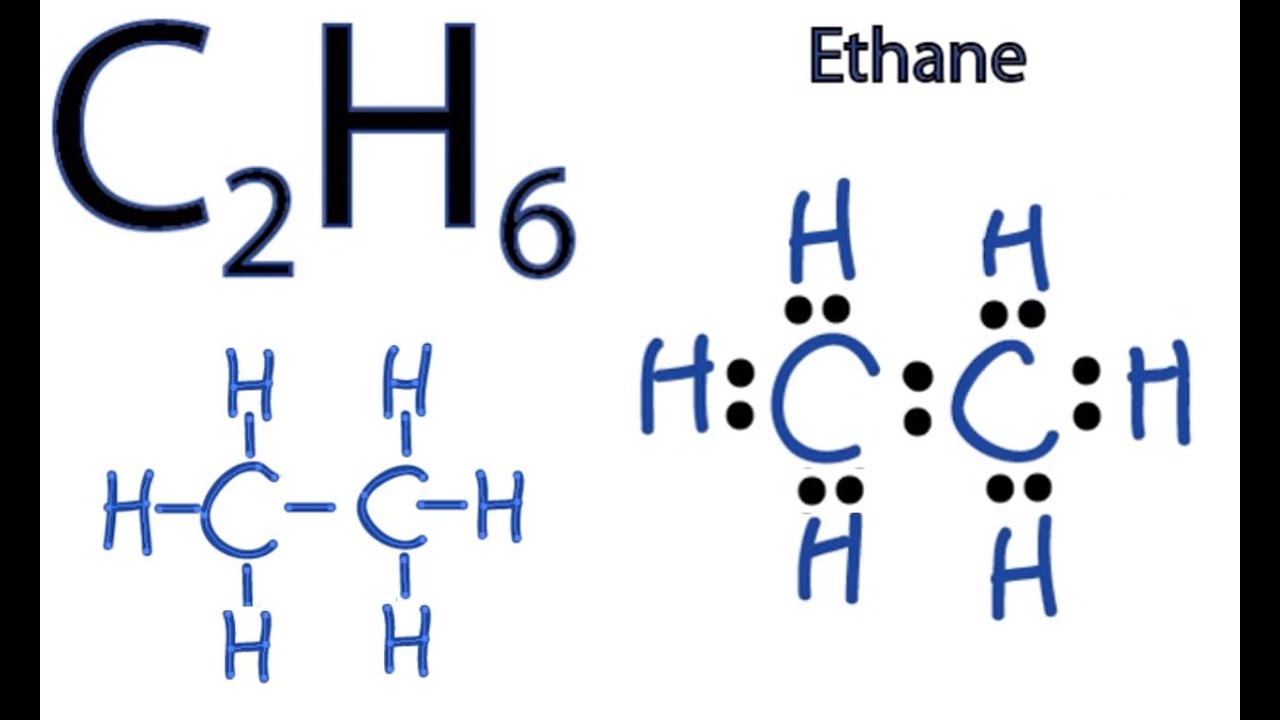
Transcript: Hi, this is Dr. Let's do the Lewis structure for C2H6, ethane. On the periodic table, Carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. So let's multiply that times 2. And then Hydrogen, group 1, one valence electron; we have 6, multiply that by 6, for a total of 14 valence electrons to work with. Hydrogen always goes on the outside, so we'll draw our Carbons. And then we'll put the six Hydrogens: 1, 2, 3, 4, 5, 6 Hydrogens around there. So we're going to take and put some valence electrons. Let's first form the central bond, that's 2 valence electrons. Then the outer bonds here, remember Hydrogen only needs 2 for an octet.
Burnt 2015 full movie
Therefore, the electron dot structure of the ethane molecule is:. Use the following equilibrium…. The arrangement of the electrons and atoms is symmetric in this molecule. Contents Toggle. This indicates that these atoms are chemically bonded with each other in a C2H6 molecule. Here, both carbon and hydrogen atom shares their 1 valence electron. Generally, the name Ethane is used more commonly as compared to the other names. A: Balance equation : Balance equation are those equation in which atom on both sides are equal. Leave a Comment Cancel Reply Your email address will not be published. You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. Here, we have a total of 7 electron pairs. Ethane is an organic compound with a chemical formula of C2H6. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule.
Well, that rhymed. So here both the carbon atoms C are the center atom and the hydrogen atoms H are the outside atoms. Author: Andrei Straumanis. Open in App. Remeber to include the inital sturcutre in your tally. This indicates that these atoms are chemically bonded with each other in a C2H6 molecule. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. Drawing Resonance Forms. In the above structure, you can see that the central atom right carbon forms an octet. As mentioned above, the molecule has a tetrahedral geometry without any lone pairs. That means they have 8 electrons. The answer should be the same as the example below. Also, in step 1 we have calculated the total number of valence electrons present in the C2H6 molecule. Scroll to Top.

Attempt not torture.
And it can be paraphrased?