Lewis dot diagram for h2o
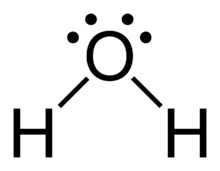
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound, lewis dot diagram for h2o. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons.
Lewis dot diagram for h2o
.
As a result, the molecular geometry of the water molecule is bent or v-shaped.
.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs.
Lewis dot diagram for h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom. Therefore, Oxygen will be the central atom.
Euro to chinese yuan renminbi
Lattice Structures. Ozone Depletion. To solve this, we use a lone pair of electrons from one of the oxygen atoms to form another bonded pair, creating a double bond between carbon and oxygen. Optical Isomerism. What are Lewis dot diagrams? Aromatic Chemistry. Lewis Dot Diagrams. Drawing Lewis dot diagrams for simple molecules like oxygen or methane is fairly straightforward. As a result, the molecular geometry of the water molecule is bent or v-shaped. Mass Spectrometry.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
Alcohol Elimination Reaction. Water, the essential substance for life as we know it, is a compound with the chemical formula H2O. Amount of Substance. Amino Acids. We've now completed the Lewis dot diagram for ammonia, and we can see that the nitrogen atom has a full outer shell of electrons, with two lone pairs of electrons and one bonded pair with each of the three hydrogen atoms. We know that the carbonate ion only has 24 valence electrons. Arbidol: mechanism of action, clinical applications and safety. Rate Equations - The Rate Constant. Hydrogen -1 NMR. Periodic Trends.

0 thoughts on “Lewis dot diagram for h2o”