Lewis dot structure for becl2
BeCl2 lewis structure has a Beryllium atom Be at the center which is surrounded by two Chlorine atoms Cl. There are 2 single bonds between the Beryllium atom Be and each Chlorine atom Cl.
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum. The molar mass and melting point of beryllium chloride are
Lewis dot structure for becl2
.
So you have seen the above image by now, right?
.
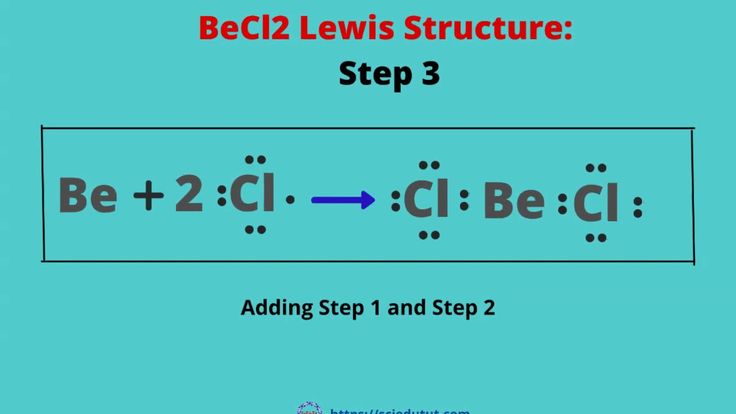
Drawing BeCl2 Lewis Structure is very easy. Here in this post, we described step by step method to construct BeCl2 Lewis Structure. A three-step approach for drawing the BeCl2 Lewis structure can be used. The first step is to sketch the Lewis structure of the BeCl2 molecule, to add valence electron around the Beryllium atom; the second step is to valence electron to the two chlorine atoms, and the final step is to combine the step1 and step2 to get the BeCl2 Lewis Structure. The BeCl2 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the BeCl2 molecule. Finally, you must add their bond polarities to compute the strength of the Be-Cl bond dipole moment properties of the BeCl2 molecule. As a result, it has no dipole moment. The BeCl2 molecule has no dipole moment due to an equal charge distribution of negative and positive charges. The central atom is beryllium, which is bordered on two terminals with chlorine atoms. Beryllium has two outermost valence electrons, indicating that it possesses two electrons in its outermost shell, whereas chlorine only has seven valence electrons in its outermost shell.
Lewis dot structure for becl2
BeCl2 lewis structure has a Beryllium atom Be at the center which is surrounded by two Chlorine atoms Cl. There are 2 single bonds between the Beryllium atom Be and each Chlorine atom Cl. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a BeCl2 beryllium dichloride molecule , first of all you should know the valence electrons present in beryllium atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Beryllium is a group 2 element on the periodic table.
Ave maria song lyrics
Now, the 3s atomic orbital of one chlorine atom will combine with the 3s atomic orbital of other chlorine atoms and provide two 3s group orbitals of the same energy. Skip to content BeCl2 referred to as Beryllium Chloride, is an inorganic compound. Trigonal bipyramidal. For example, two atomic orbitals combine to form two molecular orbitals, one is bonding and the other is antibonding. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any molecule. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum. The molecular orbital diagram of beryllium chloride is also studied. Hence, the Lewis structure of beryllium chloride can be drawn as:. Now beryllium requires only 4 electrons to become stable. It can exist in both monomeric and 1-D polymeric forms. The electronic configuration of beryllium is [He] 2s2and chlorine is [Ne] 3s23p5. Each chlorine atom completes its octet by sharing its one electron with the beryllium atom. These two orbitals are similar in symmetry and energies are also nearby. By doing so, you will get the following lewis structure of BeCl2. Its shape can easily be predicted by the following table.
Beryllium Dichloride, having a chemical formula of BeCl 2 is an inorganic and colourless compound. It dissolves in a lot of polar solvents. BeCl 2 is used in the electrolysis reactions for Beryllium.
So here the beryllium atom Be is the center atom and the chlorine atoms Cl are the outside atoms. It appears as white or yellow crystal solid at room temperature. Therefore, the hybridization of the Beryllium atom in the Beryllium chloride is sp. Chlorine is group 17 element on the periodic table. November 28, Now, there are two atoms, which are bonded to the Beryllium atom and there are no lone pair of electrons at the Beryllium atom. The same can be represented by its orbital diagram. The electronegativity of Both Chlorine atoms is the same and thus has equal influence on the shared electrons. BeCl2 Polarity. The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The molecular orbital diagram of the BeCl2 molecule is drawn by the combination of Beryllium atomic orbitals and chlorine group orbitals. There are 3 lone pairs on both the Chlorine atoms Cl.

Absolutely with you it agree. In it something is also idea excellent, I support.
You are right, in it something is. I thank for the information, can, I too can help you something?