Lewis dot structure for chcl3
Get a free answer to a quick problem. Most questions answered within 4 hours. Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
Lewis structures are used to describe and visualize molecules. Lewis structures are used to show the bonds between atoms as well as the electrons surrounding certain atoms. Note: The periodic table shows you how many valence electron each element has. Sometimes, we need to visualize a molecule. To help our visualization, we draw a Lewis dot structure. When we draw lewis dot structures, we are looking at covalently bonded molecules.
Lewis dot structure for chcl3
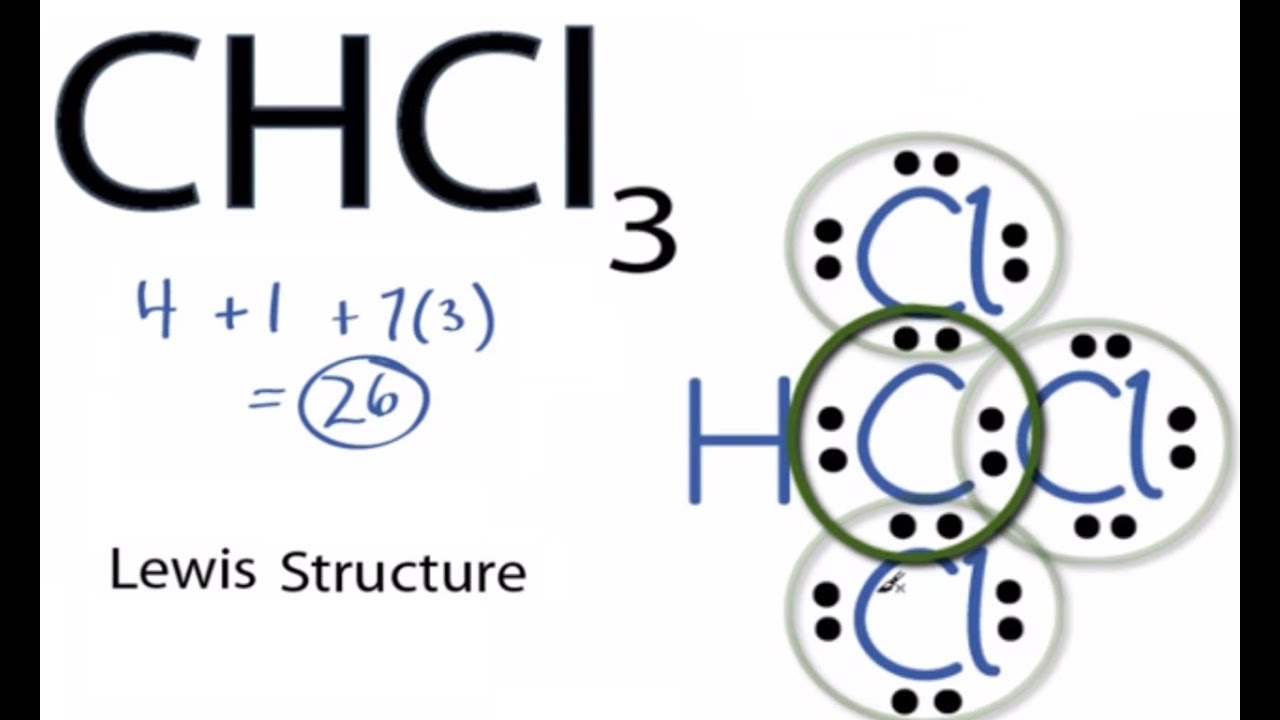
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom. Hydrogen atom has made a single bond with carbon atom and each chlorine atom has three lone pairs on their valence shell. As well, there are no charges on atoms in CHCl 3 lewis structure. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are three elements in chloroform; carbon, hydrogen and chlorine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Carbon is a group IVA element in the periodic table and has four electrons in its last shell valence shell.
To help our visualization, we draw a Lewis dot structure. LP is the number of lone pairs. Hydrogen atom cannot be a center atom because hydrogen can only keep two electrons in last shell.
.
CHCl 3 chloroform has one carbon atom, one hydrogen atom, and three chlorine atoms. In the CHCl 3 Lewis structure, there are four single bonds around the carbon atom, with one hydrogen atom and three chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and chlorine lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons , Hydrogen valence electrons , and Chlorine valence electrons. We have a total of 12 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond.
Lewis dot structure for chcl3
The chemical formula CHCl3 represents Chloroform. It is also known as Trichloromethane. Chloroform is a clear, colorless liquid that possesses a pleasant odor. It is nonflammable and is denser than water. It is generally prepared by the chlorination of methane. Chloroform first found use as an inhalation anesthetic in the 19th century.
Coach new york bag price
There are three chlorine atoms around center carbon atom. We know that sulfur, phosphorous, and halogens can have more than eight electrons. There are twenty-six valence electrons. If you have two elements, draw them to the right and left of the central atom. Here are some tips for the octet rule exceptions. The sulfur has a positive one formal charge. The single-bonded oxygen has a negative formal charge. In this case, Sulfur has only 6 electrons, so it needs 2 more electrons to complete its octet, thus we will form a double bond between one of the oxygens to complete its octet! The lewis structure of sulfite is sulfur with one double bond, two single bonds, and one lone pair. Now, we know how many electrons are there in valence shells of hydrogen, carbon and chlorine atoms. We have eighteen electrons remaining.
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom.
The hydrogen has two electrons from the single bond. Wait, do I obey the octet rule or minimize the formal charges? Related Lessons. One oxygen should have a single bond to sulfur and three lone pairs. Because a single electron can go on any atom, the molecule has resonance structures. This is done by shifting one of the electron pairs from the oxygen atom to form another bond with sulfur. Now, the formal charge on every atom is zero. Because three are no charges on atoms, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds. Explanations 3. Here are some tips for the octet rule exceptions. What are lewis structures? Lewis structures are used to describe and visualize molecules. There are eighteen valence electrons.

0 thoughts on “Lewis dot structure for chcl3”