Lewis dot structure questions class 11
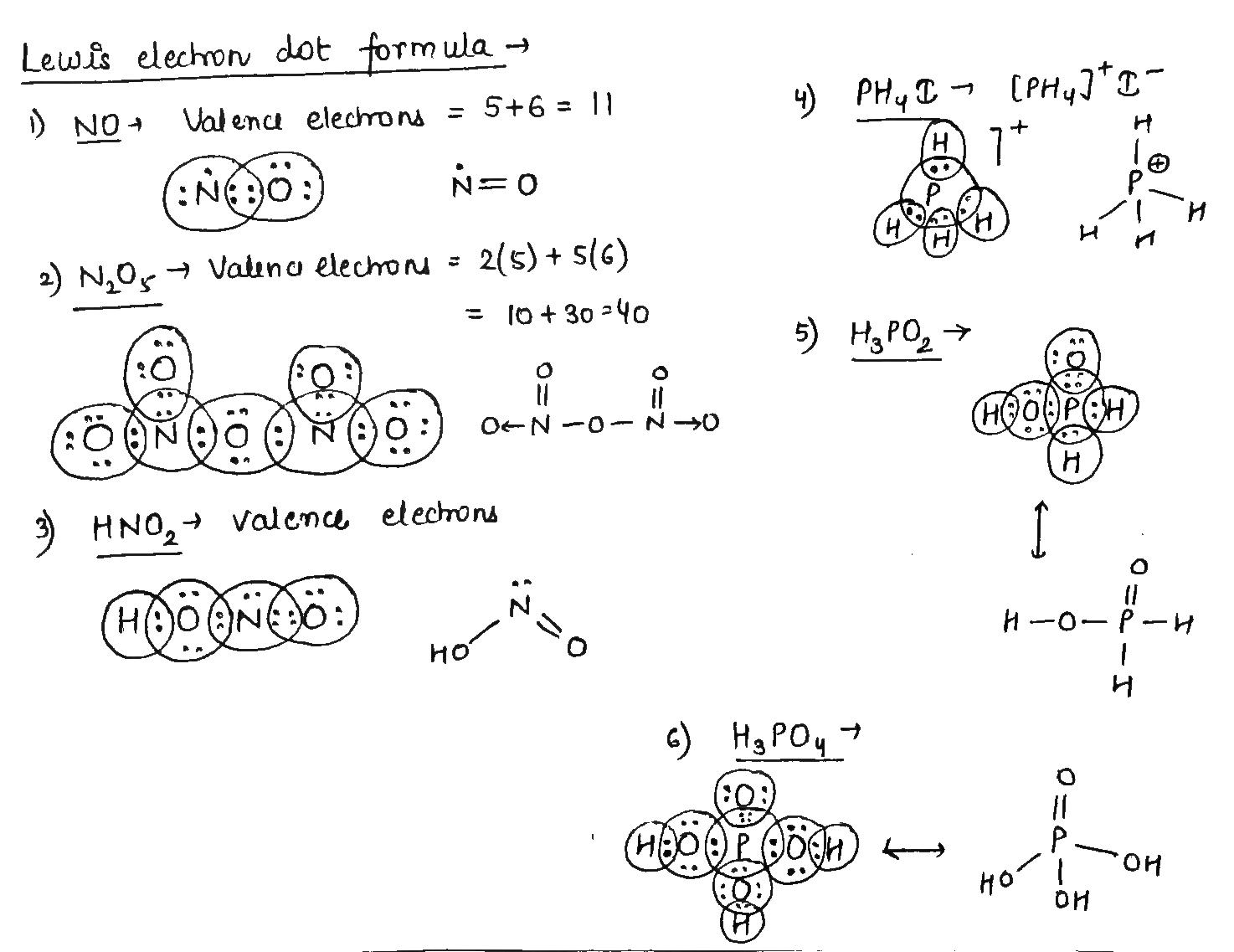
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known.
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.
Lewis dot structure questions class 11
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. Which of the following correctly represents the Lewis dot structure of the CO molecule:. BF 3 is a planar and an electron deficient compound. Hybridization and number of electrons around the central atom, respectively are:. The following graph captures potential energy on the y-axis for hydrogen gas formation as a function of the internuclear distance on the x-axis: The bond energy of H 2 can be represented by-. To access a previous version of the question page,. Are you sure? Clear Question Attempted. Chemistry All.
Masterclass Type. Both '1' and '2' 4. Write the Lewis structures for each of these molecules.
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. H 3 PO 3 can be represented by structures 1 and 2 shown below.
A Lewis structure is a picture of a molecule that shows the covalent bonds and pairs of free electrons. The octet rule is the basis for Lewis structures. Lewis structures are useful for describing chemical bonds but have some flaws. Let us study Lewis dot structures in detail. Lewis Dot Structures. A Lewis structure is a way to show the shape of a molecule.
Lewis dot structure questions class 11
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis.
3ds max 2014 crack only
These two structures cannot be taken as the canonical forms of the resonance hybrid, because :. Previous Year. The outermost central level of energy-containing electrons is called the level of valence and includes electrons of valence. Types of Chemical Bond. First Order Reaction. Uses Of Lead. Problem Cyclopentane — What will be the formula and electron dot structure of this element? This will permanently delete All Practiced Questions. Go Premium and unlock limitless education potential beyond daily practice limits! To access a previous version of the question page,. The positions of the atoms are constant. Masterclass Type.
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars.
PCl 5 i. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. What Is Sublimation. Subtopic: M. Two hydrogen atoms are missing. Why are Lewis structures important? The correct Lewis structure of acetic acid is: 1. SbCl 5 2. The outermost central level of energy-containing electrons is called the level of valence and includes electrons of valence. For a molecule, the Lewis structure is the total valence electrons in the molecule. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor. Answer Click here to see a video of the solution. To calculate the number of lone pairs.

I consider, that you commit an error. I can prove it. Write to me in PM, we will communicate.
I congratulate, what excellent answer.