Lewis structure for no2+
The nitrogen and oxygen elements come as the member of the nitrogen and oxygen family lewis structure for no2+ from the periodic table respectively. The valence electrons in nitrogen and oxygen are five and six respectively. The branch of nitronium ion chemistry is used to make chemicals reagents for nitrating reactions.
Step 1: The central atom will be the N atom since it is the less electronegative. Connect the N with the O atoms with single bonds:. Step 2: Calculate the of electrons in p bonds pi bonds, multiple bonds using formula 1 in the article entitled Lewis Structures and the Octet Rule. Alternatively, 1 triple bond is added between N and O. Unshared electron pairs are added so that there is an octet of electrons around each atom.
Lewis structure for no2+
As with all other Lewis Dot Structures the bonds within the structure can be replaced by two dots. The presence of this positive charge induces a quite strong attraction for the molecule as a whole to balance this out either with a negatively charged cation or with an extra electron. In fact the NO2 neutral species with an extra electron tacked onto the nitrogen is quite unique in its stability when compared to similar molecules with electrons lacking a pair. As the Lewis Dot Structure reveals, the compound would not exist in a stable form and requires negative charge to balance it out. This can often be supplied by a negative cation such as PF6-, forming a stable nitronium salt. These salts are most often utilized in order to add NO2 species to other molecules such as the benzene rings shown in the diagram above. Researchers in that lab attempted to add NO2 species to benzene rings under different acidic conditions to understand which would be most effective at producing different results. If you are interested in learning more about this subject, your in luck! The interesting properties result from the complex interaction between its structure as determined by polarity and its status as a cation. Labels: chemistry , Lewis Structures , Science , zlatest. Newer Post Older Post Home. Subscribe to: Post Comments Atom.
The nitrogen atom has an electronegativity value of 3. Alternatively, 1 triple bond is possible between C and N atoms. As with all other Lewis Dot Structures the lewis structure for no2+ within the structure can be replaced by two dots.
.
The Lewis structure is a structure that shows the bonding between atoms as short lines some books use pairs of dots , and non-bonding valence electrons as dots. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here:. For simple diatomic molecules , combining the Lewis symbols of each element gives its Lewis structure. H 2 example : H only needs two electrons; usually referred to as a duet. Special note: Non-bonding electrons can also be unpaired single electrons. A species with one or more unpaired single electrons is called a radical free radical. More examples of radicals with single electrons will be in section 1. For more complicated polyatomic molecules and ions , the Lewis structures cannot be obtained by simply combing Lewis symbols.
Lewis structure for no2+
It is one of the most common gaseous molecules having a reddish-brown hue. It can be cooled and compressed into a yellowish-brown liquid for tendshipping and transport. A highly toxic poisonous chemical compound, NO2 is a major air pollutant and belongs to the group of oxides of nitrogen.
Nintendo gameboy first generation
Step 1: The central atom will be the C atom since it is the less electronegative H is a terminal atom so it cannot be a central atom. Put these values for the nitrogen atom in the formula above. Then the total outermost valence shell electrons can be calculated as follows. Connect the N with the O atoms with single bonds:. If you are interested in learning more about this subject, your in luck! The central atom is nitrogen, which is bordered on two terminals with oxygen atoms in linear geometry , and no lone pairs on the central nitrogen atom in the linear molecular geometry. Leave a Comment Cancel Reply Your email address will not be published. Lewis Structures and the Octet Rule. The valence electrons in nitrogen and oxygen are five and six respectively. It is essential for life on earth. Save my name, email, and website in this browser for the next time I comment.
The nitrogen and oxygen elements come as the member of the nitrogen and oxygen family groups from the periodic table respectively. The valence electrons in nitrogen and oxygen are five and six respectively. The branch of nitronium ion chemistry is used to make chemicals reagents for nitrating reactions.
Unshared electron pair lone pair is added so that there is an octet of electrons around each atom. Newer Post Older Post Home. The nitrogen atom has an electronegativity value of 3. Second, place the valence electron on the oxygen atoms. This can often be supplied by a negative cation such as PF6-, forming a stable nitronium salt. The presence of this positive charge induces a quite strong attraction for the molecule as a whole to balance this out either with a negatively charged cation or with an extra electron. Different kinds of nitrated benzene rings. Need to remember that, if you follow the above-said method, you can construct molecular dot structure very easily. Learn how your comment data is processed. As the Lewis Dot Structure reveals, the compound would not exist in a stable form and requires negative charge to balance it out. The total valence electron in a nitrogen atom is 8. It is one of the main essential micronutrients in plants growth. Put these values for the nitrogen atom in the formula above. But the positive charge is on the central nitrogen atom. The molecule polar behaves in a different manner as compared to nonpolar.

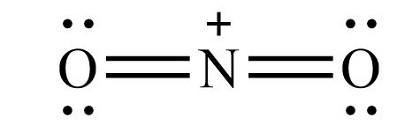
It does not approach me. Perhaps there are still variants?