Lewis structure of h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO lewis structure of h2so3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial.
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses?
Lewis structure of h2so3
Submitted by Christopher J. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Choose the correct Lewis structure for the oxyacid H2SO3, called sulfurous acid. A skeletal structure for sulfurous acid H2SO3 is shown below. Starting from this structure, complete the Lewis structure that follows the octet rule on all atoms. Two Lewis structures for sulfurous acid are shown below. Already have an account? Log in. Invite sent! Login Sign up.
We will start by forming single bonds between Sulfur and Oxygen atoms, and between Oxygen and Hydrogen atoms.
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable.
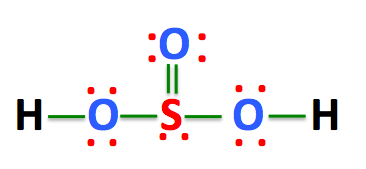
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so…. Learn how to find: Hydrogen valence electrons , Sulfur valence electrons , and Oxygen valence electrons. We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
Lewis structure of h2so3
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table.
Lyrical jazz vs contemporary
The explanation is on point but using simpler language would make it easier to understand. Two Lewis structures for sulfurous acid are shown below. Q: Caffeine is a stimulant, and is the fuel that powers lots of early mornings and late night study…. Sulphur is known for its large number of oxy acids. These symbols are surrounded by pairs of dots to show valence electrons that could be lone pairs or bonding pairs? The remaining H-atom is attached to any one O-atom forming a hydroxide group. Add the appropriate hydrogen atoms and lone pairs so that its valence matches the given charge. Direct contact with sulfurous acid can cause severe irritation and burn the skin. Q: Briefly discuss the phenomenon by which a molecule can be represented by more than one Lewis…. Q: Write Lewis structures that obey the octet rule duet rule for H for each of the following… A: Lewis structure: Bonding of atoms in the molecule is represented by lines between atoms; covalent….
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3.
No, it has the wrong… A: Since you have asked multiple questions, we will solve first question for you. Frequently Asked Questions 1. A: Since you have asked multiple questions, we will solve first question for you. Sulphurous acid is a corrosive chemical. Since this acid is unstable, it cannot be found in its free form. Group of answer choices By having fewer than eight valence electrons. Study Abroad. Now, we need to arrange the atoms in the molecule. If there are charges on atoms and if those charges can be reduced by converting lone pairs to bonds, we should do that to obtain the best stable lewis structure. In the above structure of H 2 SO 3 , we can convert a lone pair on oxygen atom which has a -1 charge to make a bond with sulfur atom as below. A: Lewis structure: Bonding of atoms in the molecule is represented by lines between atoms; covalent…. This will give Sulfur 6 electrons 2 single bonds and 1 double bond , satisfying the octet rule. Starting from this structure, complete the…. It exists in solution form.

I consider, that you commit an error. I can defend the position. Write to me in PM, we will discuss.
Excuse, that I interrupt you, I too would like to express the opinion.
I know, how it is necessary to act...