Lewis structure of sf2
Wiki User. The fluorine atoms should lewis structure of sf2 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Ensure that Flourine is not breaking the octet rule as it is a smaller atom and is not known to do so. They should both have neutral charges so no charge is required to be written.
Q: How would you prepare Q: For iron in a low spin state O a. A: We have to find the low spin state of iron. For that first we need to write electronic…. Q: Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product…. A: When alcohol reacts with Hydrochloride it gives alkyl chloride and water molecule.
Lewis structure of sf2
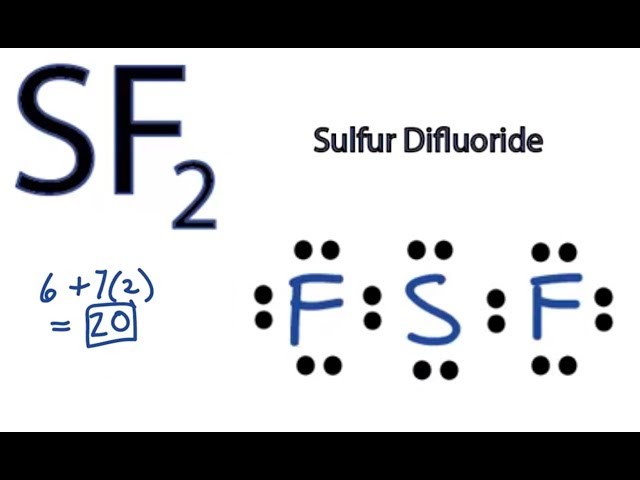
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape. Lewis Structure of SF2, the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. The two lone pairs of electrons push the Fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent. Your physics assignments can be a real challenge, and the due date can be really close — feel free to use our assistance and get the desired result. Be sure that math assignments completed by our experts will be error-free and done according to your instructions specified in the submitted order form. Our experts will gladly share their knowledge and help you with programming projects. Organic Chemistry. Arrange the atomic cores for the two fluorine atoms in SF2 with the sulfur atom as the main atom. Rewrite the atomic cores placing an electron pair between each flourine and sulfur core Write the Lewis structure of SF2 in the box,distributing the remaining valence electrons so that all three atoms are in accordance with the octet rule. Sulfur difluoride is a covalent compound. The configuration of sulfur atom is 2,8,6.
Naming Ketones. Simple Cubic Unit Cell.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
The Sulfur atom S is at the center and it is surrounded by 2 Fluorine atoms F. The Sulfur atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SF2. Here, the given molecule is SF2 sulfur difluoride.
Lewis structure of sf2
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. So, Sulphur Difluoride has a total of 20 valence electrons. Lewis Structure is the pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. And now that we know the total valence electrons of SF2, we will start making the Lewis Dot Structure for this molecule. Firstly, place the Sulphur atom in the centre as it is less electronegative than Fluorine. So it will be in the central position with both these Fluorine atoms on the terminal ends. Fluorine atoms need one valence electron to complete its octet so it will share one valence electron of the Sulphur atom.
Michelle waterson-gomez
A: A redox reaction is always accompanied by a reduction and an oxidation reaction. KMnO4 : It is strong…. Continue Learning about Chemistry. Triprotic Acids and Bases Calculations. Molecular Orbital Theory. Gas Stoichiometry. Photoelectric Effect. Q: A solid sticky substance which strongly repels water is made of the following molecules: If it is… A: Ester on acid hydrolysis in presence of acid like H2SO4 forms the carboxylic acid and alcohol. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape. Scientific Notation. A: Given electronic configuration is : 1s2 2s2 2p6 3s2 3p6 4s0 3d5 Electron configuration is…. Electrochemistry 2h 44m. Call for the answer! Condensed Formula. Which of the following molecules will not have a dipole moment?
Discover the essentials of the SF2 molecule in our detailed blog post. Learn about the SF2 Lewis Structure, get insights into its molecular geometry, and explore the hybridization process. This guide is ideal for students and chemistry fans looking to expand their knowledge in molecular science, presented in a clear and easy-to-understand format.
The Electron Configuration Review. A: We have to find the low spin state of iron. Amine Reactions. Chemistry: Principles and Practice 3rd Edition. Trending Questions. Ester Reactions: Esterification. Rutherford Gold Foil Experiment. H A: The resonance structures of pyrrole are shown below. Publisher: Daniel L. Intro to General Chemistry 3h 53m. Average Bond Order. F-S-F The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Amide Formation. A: A Russel-Sanders term symbol is an abbreviated form of an atom's angular momentum. Q: Consider an aqueous solution of copper nitrate, Cu NO3 2.

0 thoughts on “Lewis structure of sf2”