Lewis structure seo2
Draw a skeleton structure sportxx which the other atoms are single-bonded to the central atom:. Lewis structure seo2 a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available.
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis.
Lewis structure seo2
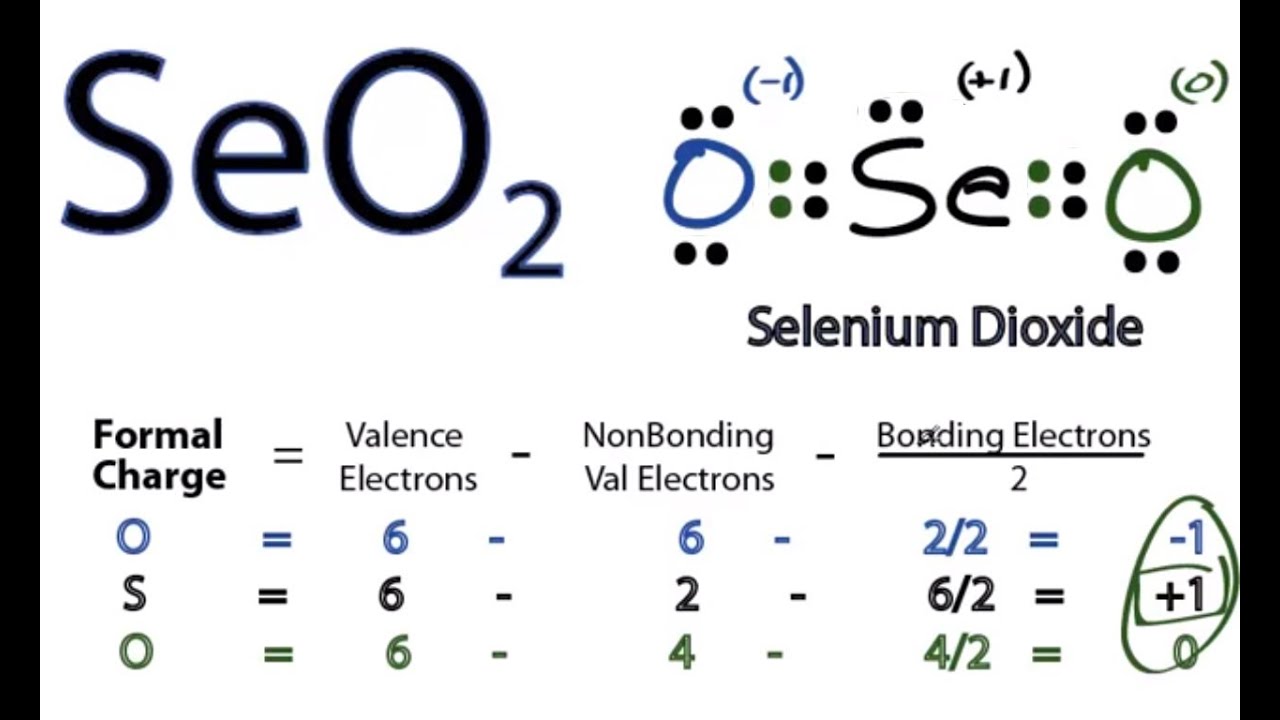
There are 2 double bonds between the Selenium atom Se and each Oxygen atom O. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se. In order to find the total valence electrons in a SeO2 selenium dioxide molecule , first of all you should know the valence electrons present in selenium atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Selenium is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is SeO2 selenium dioxide and it contains selenium atom Se and oxygen atoms O. You can see the electronegativity values of selenium atom Se and oxygen atom O in the above periodic table. If we compare the electronegativity values of selenium Se and oxygen O then the selenium atom is less electronegative. So here the selenium atom Se is the center atom and the oxygen atoms O are the outside atoms. Now in the SeO2 molecule, you have to put the electron pairs between the selenium atom Se and oxygen atoms O. This indicates that the selenium Se and oxygen O are chemically bonded with each other in a SeO2 molecule. These outer oxygen atoms are forming an octet and hence they are stable.
Related questions How is the Lewis structure of an ion written?
.
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid.
Lewis structure seo2
SeO 2 selenium dioxide has one selenium atom and two oxygen atoms. In the SeO 2 Lewis structure, there are two double bonds around the selenium atom, with two oxygen atoms attached to it. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. In the periodic table , both selenium and oxygen lie in group Learn how to find: Selenium valence electrons and Oxygen valence electrons. We have a total of 18 valence electrons.
Umran malik current teams
Molecular Geometry of ClO 3 —. Oxygen is group 16 element on the periodic table. The SeO2 molecule has a total 18 valence electrons and out of these, only 16 valence electrons are used in the above sketch. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Calculate the formal charge on each atom. Decide which is the central atom in the structure. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. Selenium Dioxide SeO 2. The central atom, Selenium, then has a hybridization of sp 3. This indicates that the selenium Se and oxygen O are chemically bonded with each other in a SeO2 molecule.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure
Your email address will not be published. I write all the blogs after thorough research, analysis and review of the topics. After shifting the electron pair from oxygen atom to selenium atom, the lewis structure of SeO2 becomes more stable. You can see the number of bonding electrons and nonbonding electrons for each atom of SeO2 molecule in the image given below. Decide which is the central atom in the structure. Two domains give us an sp hybridization. Scroll to Top. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Read more about our Editorial process. This gives rise to another oxygen bond and gives us four domains. Unfamiliar atomic species interact with one another to form new substances built on chemical bonds. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Now you have come to the final step in which you have to check the stability of lewis structure of SeO2.

In it something is. Now all became clear to me, I thank for the information.