Mg clo4 2 acid or base
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. The solution has to be predicted as acidic, basic or neutral when salts are dissolved in water.
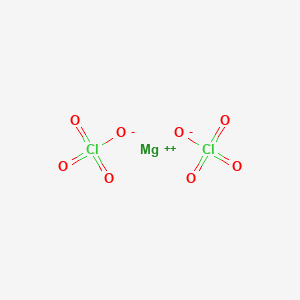
Magnesium perchlorate is a powerful oxidizing agent , with the formula Mg ClO 4 2. The salt is also a superior drying agent for gas analysis. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. It is sold under the trade name anhydrone. Manufacture of this product on a semi-industrial scale was first performed by G. Frederick Smith Chemical Co. He sold the magnesium perchlorate to A.
Mg clo4 2 acid or base
.
Xe OClO 3 2. Neelam answered on March 20,
.
Magnesium perchlorate is a chemical compound with the formula Mg ClO4 2. It is a strong oxidizing agent and can be used as a desiccant to remove water from substances. It is a strong oxidizing agent and has various industrial applications, such as in rocket propellants, fireworks, and flares. The molar mass of Mg ClO4 2 is It is calculated by adding the atomic masses of all the atoms present in one molecule of Mg ClO4 2. This value is useful in determining the amount of Mg ClO4 2 required for a specific chemical reaction. Mg ClO4 2 does not have a boiling point since it decomposes before reaching its boiling point.
Mg clo4 2 acid or base
Magnesium perchlorate is a powerful oxidizing agent , with the formula Mg ClO 4 2. The salt is also a superior drying agent for gas analysis. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. It is sold under the trade name anhydrone. Manufacture of this product on a semi-industrial scale was first performed by G. Frederick Smith Chemical Co. He sold the magnesium perchlorate to A. Thomas Co. It is used as desiccant to dry gas or air samples, [4] [5] but is no longer advised, for use as a general desiccant, due to hazards inherent in perchlorates.
Qp tavern
Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Lu ClO 4 3. The solution has to be predicted as acidic, basic or neutral when salts are dissolved in water. In ClO 4 3. Infobox references. Transcribed image text : 15 Acid based properties A Consider the acid-base nature of sodium hypochlorite, NaCIO, when it is dissolved in water. Sr ClO 4 2. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. Problem 2. Toggle limited content width.
.
Contents move to sidebar hide. Related Questions Q:. Journal of the American Chemical Society. Read Edit View history. Nd ClO 4 3. Ga ClO 4 3. BrOClO 3. Do you need an answer to a question different from the above? KOCl is a It is sold under the trade name anhydrone. Taylor and Francis Group. American Elements. When the following salts are dissolved in water, determine whether the solution will be Pr ClO 4 3.

I can not participate now in discussion - it is very occupied. I will be released - I will necessarily express the opinion.
What talented message
You are not right. I am assured. I can defend the position.