Mgcl2 is ionic or covalent
Magnesium chloride is an ionic compound while hydrogen chloride is a covalent compound.
Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. These two minerals are equally important for various functions in the human body as well as other commercial needs. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. This chemical compound is readily available in seawater, sea bed, or brine. This mineral can also be extracted by the process of solution mining and evaporation of seawater.
Mgcl2 is ionic or covalent
.
Moreover, by joining our online doubt-clearing classes, you can prepare chemistry at its best level. Due to the ionic bond of magnesium ion and chlorine ion, magnesium chloride forms.
.
MgCl 2 can be extracted from seawater or brine and is chemically named Magnesium Chloride. Chloromagnesite is non-combustible but when heated liberates irritating toxic gas or fumes. Inhaling this compound causes severe coughing. Well water consists of dissolved magnesium chloride. It was used in locomotive boilers located on the Trans-Australian Railway and resulted in serious and costly maintenance problems. This problem occurred during the steam era. There was no facility for freshwater watercourses, therefore they had to rely on bore water.
Mgcl2 is ionic or covalent
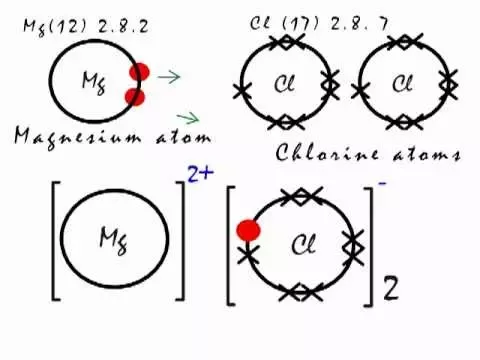
MgCl2 Magnesium chloride is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Here, Mg is a metal and Cl is a nonmetal. So when they combine, it forms an ionic compound. Well, now you have got to know that MgCl2 is an ionic compound, but let me explain the in-depth reason why MgCl2 is an ionic compound. As mentioned above, you can simply remember that when the metal combines with nonmetal, the bond between them is an ionic bond. Magnesium atom have 12 electrons. Now Magnesium is a metal and the metals are highly electropositive that means they have the tendency to lose electrons and become positive ions. Hence during the chemical reaction, the Magnesium atom will lose 2 electrons to form a stable octet. Chlorine atom have 17 electrons.
2pm cet to est
There is one particular hydrous Magnesium Chloride known as Bishofite, which can be extracted out of the ancient seabed. In M g C l 2 , Magnesium has 12 electrons, and its electronic configuration can be written as 2 , 8 , 2. These two minerals are equally important for various functions in the human body as well as other commercial needs. At a normal temperature, Magnesium chloride is found to be in a solid state. This ionic salt is one of the most important components used to control dust, wind erosion, and soil stabilization. However, as a by-product, carbon dioxide will also be produced along with water. In its solid state at room temperature, every anion is encircled by cations and vice versa. Its water solubility for anhydrous is as under:. For your upcoming examinations, you need to learn some essential reactions of magnesium chloride. North America. Magnesium Chloride Formula.
In chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements.
Colour — White, light grey, colorless. However, there are both positive and negative impacts of applying magnesium chloride to bare soil and roads. Similar Questions. Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. For hexahydrate. In North America, MgCl 2 is naturally abundant in the largest amount. Magnesium chloride flakes are effectively used as an alternative to Epsom salt or magnesium sulfate in horticulture or gardening. Ans: d. The refractive index of the magnesium chloride I anhydrous is 1. Ans: a. Magnesium Chloride Melting Point.

0 thoughts on “Mgcl2 is ionic or covalent”