Molar mass of calcium oxide
Calcium oxide formula : Ca Ocommonly known as quicklime or burnt limeis a widely used chemical compound.
Molar mass of CaO Calcium oxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Molar mass of calcium oxide
Calcium oxide, also known as quicklime or burnt lime, has the chemical formula CaO. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name. Last name. Grade Target Exam The molecular structure of calcium oxide can be represented as a simple ionic lattice. In this lattice structure, each calcium cation is surrounded by six oxygen anions, and each oxygen anion is surrounded by six calcium cations. This arrangement forms a crystal lattice. To calculate the molar mass of calcium oxide, we need to determine the atomic masses of calcium Ca and oxygen O from the periodic table. The atomic mass of calcium is approximately Since there is one calcium atom and one oxygen atom in calcium oxide, we can add their atomic masses together:. Answer: Calcium oxide is commonly known as quicklime or burnt lime. These names refer to the traditional method of producing calcium oxide by heating calcium carbonate such as limestone to a high temperature, resulting in the decomposition of the carbonate and the formation of calcium oxide.
Oxides are sorted by oxidation state.
.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion.
Molar mass of calcium oxide
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. O Std.
Masturbándose femenina
Cubic , cF8. Limestone, which contains less reactive material, is slower to react and may have other disadvantages compared with lime, depending on the application; however, limestone is considerably less expensive than lime. Authority control databases : National Germany. Inhalation may cause coughing, sneezing, and labored breathing. Answer: Calcium oxide is commonly known as quicklime or burnt lime. Molar mass of CaO Calcium oxide is Insoluble also in diethyl ether , octanol. No Yes. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Forensic Science International.
Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Chemical formula. Because of vigorous reaction of quicklime with water, quicklime causes severe irritation when inhaled or placed in contact with moist skin or eyes. Calcium oxide is also a separate mineral species with the unit formula CaO , named 'Lime'. Approximately 1. Barium chloride formula. CO 2 has one carbon atom and two oxygen atoms. The quicklime is not stable and, when cooled, will spontaneously react with CO 2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless slaked with water to set as lime plaster or lime mortar. E number. Contents move to sidebar hide. Hazard statements. In 80 BC, the Roman general Sertorius deployed choking clouds of caustic lime powder to defeat the Characitani of Hispania , who had taken refuge in inaccessible caves. It is a white, caustic , alkaline , crystalline solid at room temperature. Difference Between Orbit and Orbital.

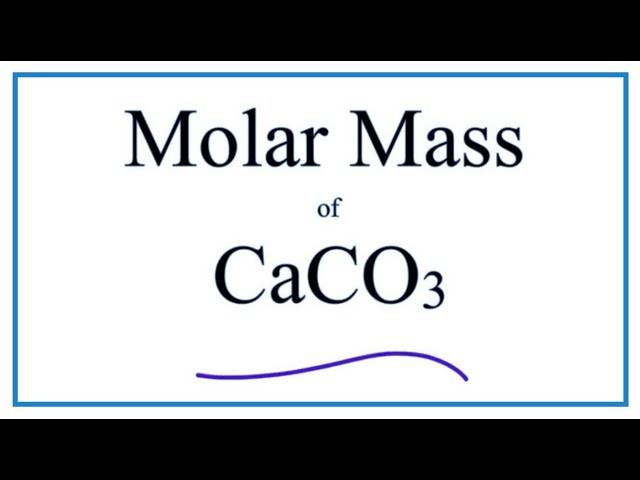
I am sorry, that I interfere, but it is necessary for me little bit more information.
By no means is not present. I know.