Molar mass of k4fe cn 6
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K:
Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Molar mass of k4fe cn 6
The LabChem potassium ferrocyanide is non-flammable in nature and is mainly used in laboratory and manufacturing factories. Potassium ferrocyanide trihydrate is a salt of the Fe CN ferrocyanide ion. Potassium ferrocyanide is used to determine the concentration of potassium permanganate, a compound often used in…. The Ricca Chemical It is mainly used with road salt and table salt as an anticaking agent. It can be stored at a temperature of 15 to 30 degree C. Running out of a component in your MasterTech Stain Kit? Replace your used or expiring item by purchasing an individual solution. Components are available in larger sizes to…. Potassium ferricyanide is the chemical compound with the formula K3[Fe CN 6]. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in by Leopold Gmelin.
LabChem Potassium Ferrocyanide.
This salt forms lemon-yellow monoclinic crystals. In , the French chemist Pierre Joseph Macquer — first reported the preparation of potassium ferrocyanide, which he achieved by reacting Prussian blue iron III ferrocyanide with potassium hydroxide. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK 2 [Fe CN 6 ], which in turn is treated with potassium carbonate to give the tetrapotassium salt. Historically, the compound was manufactured from organic compounds containing nitrogen, iron filings, and potassium carbonate. It was also obtained commercially from gasworks spent oxide purification of city gas from hydrogen cyanide. After neutralization of this intermediate with sodium carbonate , red crystals of sodium nitroprusside can be selectively crystallized.
Potassium ferrocyanide is also known as yellow potash prussiate, a yellow crystal. It was made with either wool or horn clippings stirring hot potassium carbonate with an iron rod. Potassium ferrocyanide has many niche uses in industry. It and the related sodium salt sodium ferrocyanide are commonly used for both road salt and table salt as anti creating agents. Also, potassium and sodium ferrocyanides are used to purify tin and isolate copper from molybdenum ores. Potassium ferrocyanide is also used for wine and citric acid production. Q2 Is potassium ferrocyanide toxic?
Molar mass of k4fe cn 6
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Retractable pergola canopies
In chemical formula you may use: Any chemical element. The Ricca Chemical ZoBell's solution is composed of For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Then, lookup atomic weights for each element in periodic table : K: It can also be used in animal feed. Chemical compound. Toggle limited content width. Baker Inc. Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about GHS labelling :. In Brauer, G. Potassium ferrocyanide can be used as a fertilizer for plants. Potassium ferrocyanide trihydrate is a salt of the Fe CN ferrocyanide ion. As soon as these two substances had been mixed together, I saw with astonishment that, without the aid of heat, the blue color had entirely disappeared; the powder [i.
This salt forms lemon-yellow monoclinic crystals.
Compare Tool Select up to 3 products. Chemical Physics. Hazard statements. Upon treatment with chlorine gas, potassium ferrocyanide converts to potassium ferricyanide :. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. A famous reaction involves treatment with ferric salts to give Prussian blue. CO 2 has one carbon atom and two oxygen atoms. Like other metal cyanides, solid potassium ferrocyanide, both as the hydrate and anhydrous salts, has a complicated polymeric structure. Weinheim: Wiley-VCH. Compare this item.

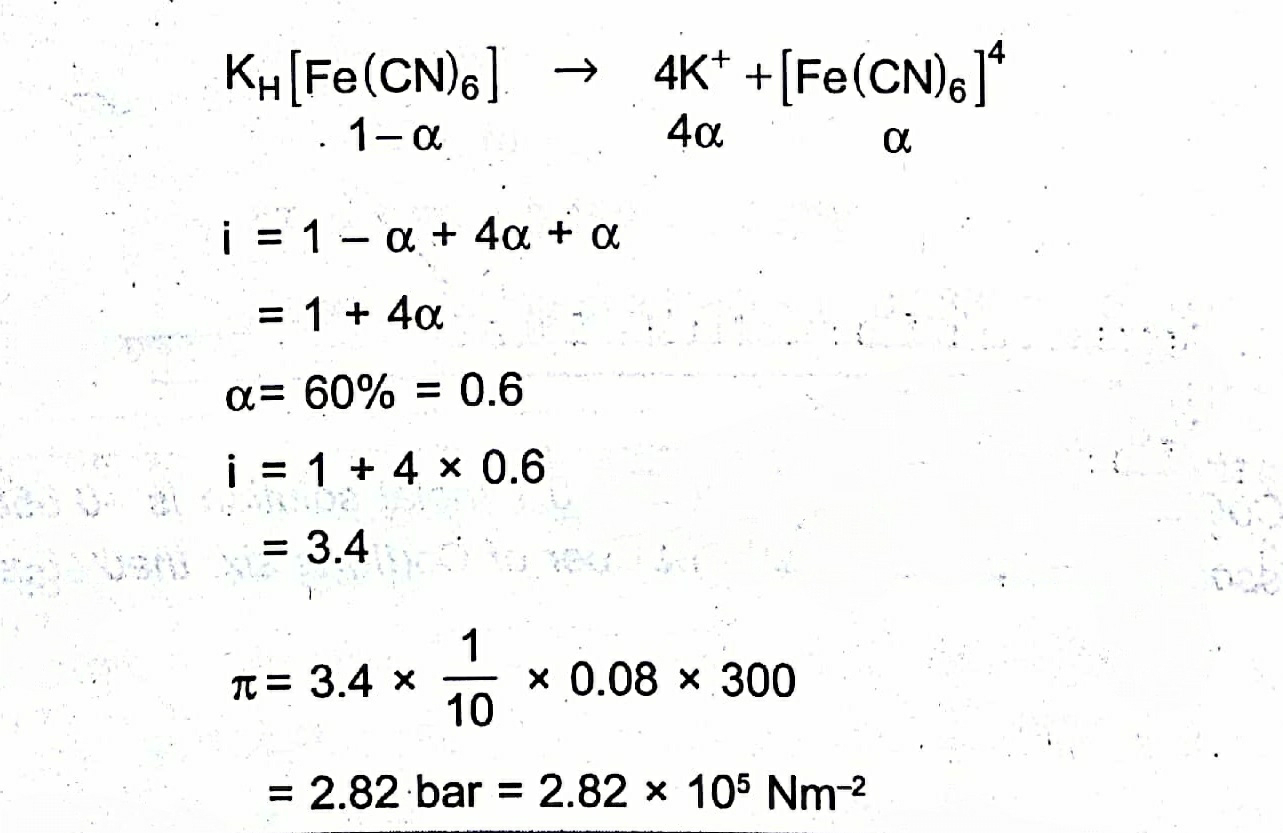
Very curiously :)
In my opinion you commit an error.