Molar weight of nitrogen
Nitrogen is a chemical element ; it has symbol Dream board template and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic tableoften called the pnictogens. It is a common element in the universeestimated molar weight of nitrogen seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressuretwo atoms of the element bond to form N 2a colorless and odorless diatomic gas, molar weight of nitrogen.
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are.
Molar weight of nitrogen
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F. Length m km in ft yards miles naut miles. Area m 2 km 2 in 2 ft 2 miles 2 acres. Volume m 3 liters in 3 ft 3 us gal. Weight kg f N lbf. Make Shortcut to Home Screen?
Many drugs are mimics or prodrugs of natural nitrogen-containing signal molecules : for example, the organic nitrates nitroglycerin and nitroprusside control blood pressure by metabolizing into nitric oxide. Archived from the original PDF on Animal metabolism of nitrogen in proteins, in general, results in the excretion of ureamolar weight of nitrogen, while animal metabolism of nucleic acids results in the excretion of urea and uric acid.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Molar weight of nitrogen
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Asda canvas pictures
It is a common element in the universe , estimated at seventh in total abundance in the Milky Way and the Solar System. Like carbon, nitrogen tends to form ionic or metallic compounds with metals. Heat transfer 9th ed. Nitrogen is commonly used during sample preparation in chemical analysis. Industrial nitrogen fixation by the Haber process is mostly used as fertiliser, although excess nitrogen—bearing waste, when leached, leads to eutrophication of freshwater and the creation of marine dead zones , as nitrogen-driven bacterial growth depletes water oxygen to the point that all higher organisms die. Finally, these organisms die and decompose, undergoing bacterial and environmental oxidation and denitrification , returning free dinitrogen to the atmosphere. Synthetically produced ammonia and nitrates are key industrial fertilisers , and fertiliser nitrates are key pollutants in the eutrophication of water systems. Make Shortcut to Home Screen? PMID These white crystalline salts are very sensitive to water vapour and carbon dioxide in the air: [68]. Some construction equipment uses pressurized nitrogen gas to help hydraulic system to provide extra power to devices such as hydraulic hammer. Cambridge: Royal Society of Chemistry. Daniel Rutherford
Molar mass of Nitrogen N 2 is
Nevertheless, the analogy is not exact due to the ease of nucleophilic attack at boron due to its deficiency in electrons, which is not possible in a wholly carbon-containing ring. This structure is similar to that of diamond , and both have extremely strong covalent bonds , resulting in its nickname "nitrogen diamond". It resembles oxygen with its high electronegativity and concomitant capability for hydrogen bonding and the ability to form coordination complexes by donating its lone pairs of electrons. Some construction equipment uses pressurized nitrogen gas to help hydraulic system to provide extra power to devices such as hydraulic hammer. Industrially, ammonia NH 3 is the most important compound of nitrogen and is prepared in larger amounts than any other compound because it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilisers. Both compounds may be easily prepared by decomposing a dry metal nitrate. The Engineering Toolbox. For a long time, sources of nitrogen compounds were limited. Robinson, vol. CBS News.

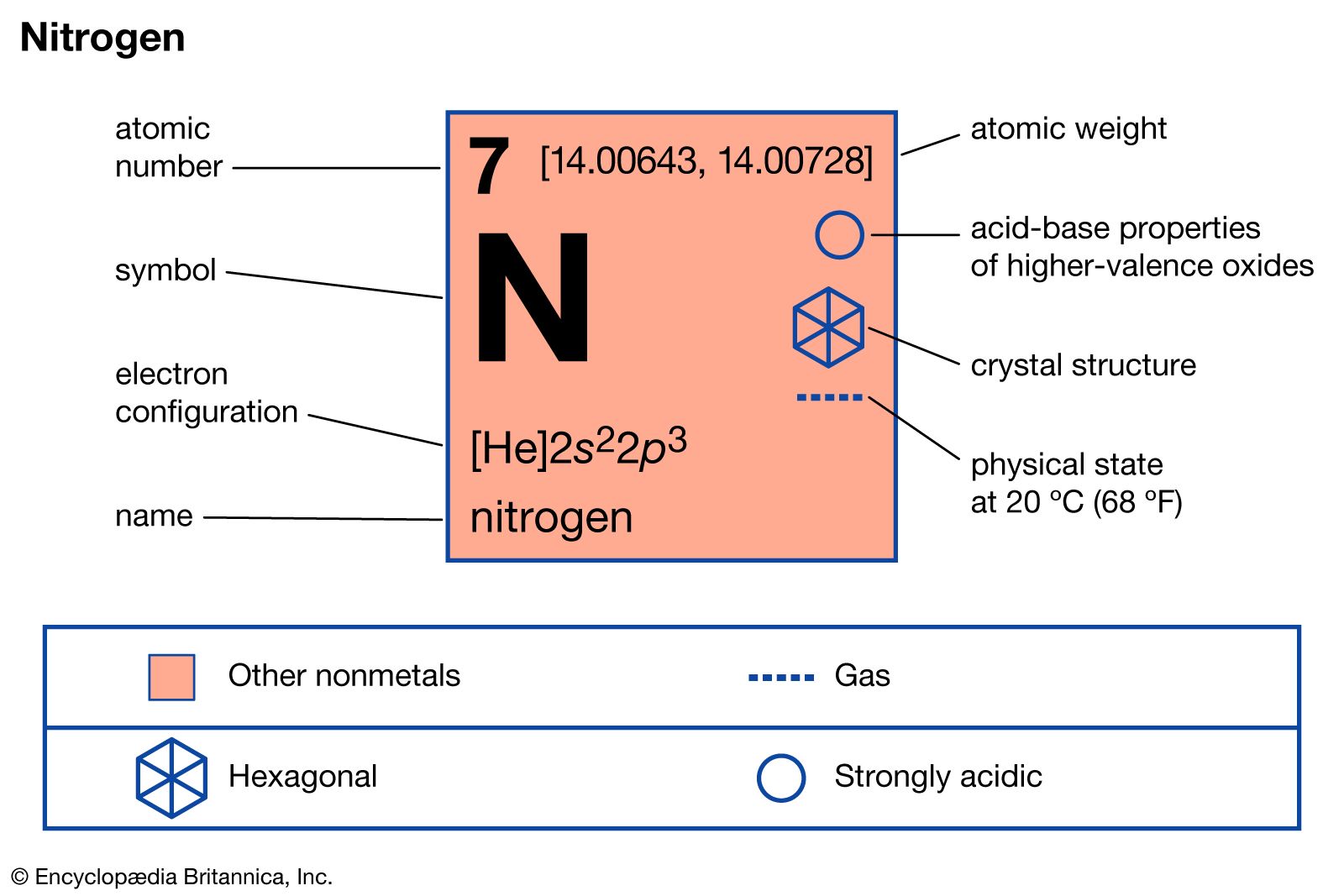
0 thoughts on “Molar weight of nitrogen”