Molecular formula of ethyne
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6.
The structural formula of ethyne is? Find the answer to this question and access a vast question bank that is customised for the student. The molecule formula for Ethyne — C 2 H 2. The elements present in ethyne are carbon and hydrogen. Atomic number of carbon — 6.
Molecular formula of ethyne
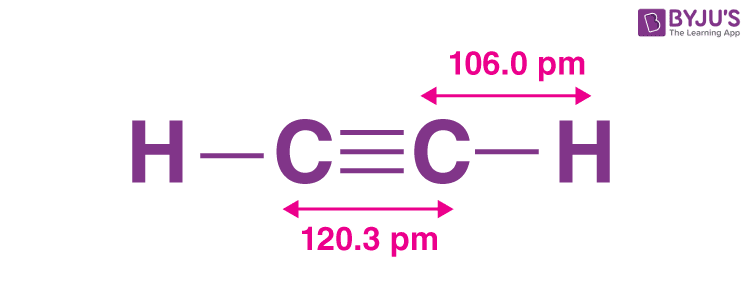
In Chemistry, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkynes , which has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics. Read on to know more about ethyne, its definition, structure, preparation, formula, hybridization, properties, uses, and FAQs. It is the simplest alkyne that exists in the form of a gas. Pure acetylene is a colourless gas with a pleasant smell. However, it sometimes contains minute amounts of stinky gas phosphine, which has a garlic-like odour. Ever since its discovery, it has been used as a fruit ripening gas, and fuel source of oxyacetylene-lamp employed in welding and cutting of metals. In ethyne, the two carbon atoms are linked together with the help of a triple bond, and the hydrogen atoms are connected to each carbon atom via a single covalent bond. As a result, the two carbon atoms contain one sigma and two pi-bonds between each other. The bond length of C-C and C-H is
The molecular formula ethyne is C 2 H 2.
Explanation: Chemical formula of Ethyne is C 2 H 2 , it has a triple bond and two single bonds. Hence, the answer is a. Menu Categories. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles Draw the electron dot structure of ethyne and also draw its structural formula.
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C 2 H 2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. Ethyne is regarded by many to be the simplest alkyne since it consists of only two carbon atoms, which are triply bonded to each other. Pure ethyne is known to be highly unstable. Therefore, it is not uncommon for ethyne to be handled in a solution. Ethyne or acetylene is an unsaturated hydrocarbon since it contains a carbon-carbon triple bond. Ethyne can be prepared by subjecting methane to partial combustion. This compound can also be prepared from the hydrolysis of calcium carbide a chemical compound with the formula CaC 2 , also known as calcium acetylide. The chemical equation for this reaction between calcium carbide and water is provided below.
Molecular formula of ethyne
Explore the properties, production methods, uses, and safety concerns of ethyne acetylene , a versatile hydrocarbon. Ethyne, also known as acetylene, is a simple aliphatic hydrocarbon. Its chemical formula is C 2 H 2 , making it the simplest member of the alkynes, a group of hydrocarbons that contain a carbon-carbon triple bond. The structure of ethyne consists of two carbon atoms triple-bonded to each other, with each also bound to a hydrogen atom.
Justwatch canada
Excited electronic configuration of carbon 1 s 2 2 s 1 2 p x 1 2p y 1 2p z 1. Learn more about the polar character of covalent bond , here. Text Solution. Acrylic acid derivatives are made by converting acetylene into acrylic acid. It is the simplest alkyne and a hydrocarbon. The solubility is 51 g for the same amount of dimethylformamide DMF. Your browser does not support the audio element. The molecular formula of carbon dioxide is CO 2. Write the structural formula of propyne. Tutorialspoint Simply Easy Learning. Therefore, the structural formula of ethyne is as follows;. There are four atoms present in an ethyne molecule including two carbon and two hydrogen atoms. Each CH molecule will result in the formation of two hybridized sp orbitals, for a total of four sp hybridized orbitals.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together.
Write the structural formula of butane.. Usage of ethyne Welding using acetylene has become far less common. Alkynes have the most common triple bond, between two carbon atoms. Testbook offers thoughtful advice from experts, sample test sets, and hand-picked study materials. The molecule formula for Ethyne — C 2 H 2. Ever since its discovery, it has been used as a fruit ripening gas, and fuel source of oxyacetylene-lamp employed in welding and cutting of metals. Structural formula of benzene is" The correct structural formula of butanoic acid is" Write the structural formula of propene. Home Chemistry Ethyne. The reaction can be given as;. Old search 2.

Excuse, topic has mixed. It is removed