Molecular geometry h2o
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecular geometry h2o, we focus on the oxygen atom.
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles.
Molecular geometry h2o
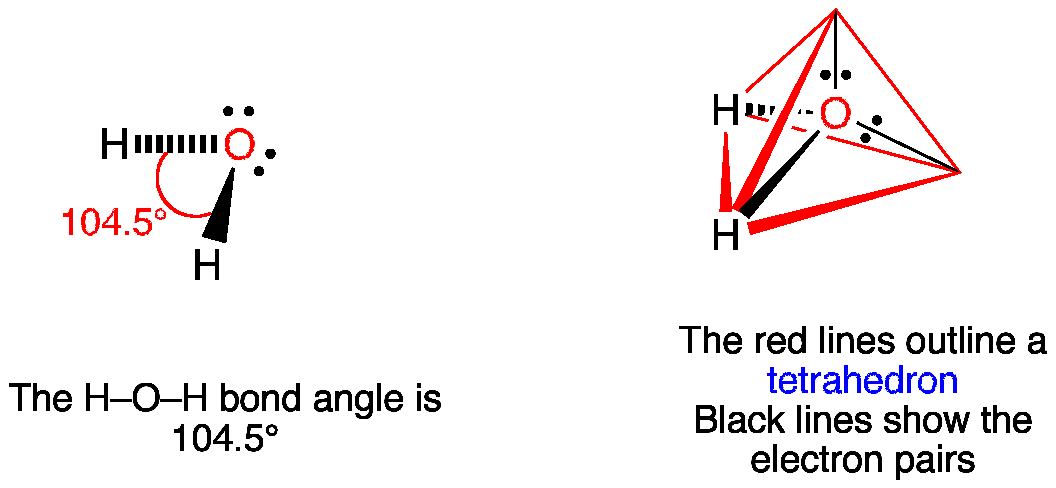
The angle between the bonds of hydrogen with oxygen are between It has a V-shaped geometry and is categorised under the tetrahedrons. Water is the most abundantly found resource, as all the oceans, seas, rivers, and water bodies comprise of water. When we first begin to study, we get to know that the chemical formula of water is H2O. This means that in a single water molecule there are two hydrogen atoms and one oxygen atom. Another name for the water molecule is Dihydrogen Monoxide consisting of two hydrogen atoms and one oxygen atom Like all other chemical compounds, water too has bonds and here we will discuss the geometry and angles that the water compound makes. The structure is scientifically known as the Lewis Structure and popularly named the Electron-dot Structure which means that it represents the molecule of water diagrammatically. This in turn makes the number of valence electrons or the electrons in the last shell of the atom known. The number of valence electrons makes sure the number of electrons that are free to create bonds and then a compound. The two-electron pairs are situated at the vertices of the molecule. These pairs are lone as they do not form a bond with the adjacent hydrogen atoms.
Allotment of Examination Centre.
The electronic geometry gives water a tetrahedral shape. The molecular geometry gives water a bent shape. Electronic geometry takes into account the electron pairs that are not participating in bonding , and the electron cloud density. Here the 2 bonds of hydrogen count as 2 electron clouds, and the 2 electron pairs count as another 2, giving us a total of 4. Molecular geometry looks at only those electrons that are participating in bonding. So here, only the 2 bonds to H are taken into account.
The oxygen atom has six valence electrons, sharing two with hydrogens each contributing one electron to complete its octet, resulting in a bond angle of Water H2O is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. The Lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties. Valence electrons are the electrons in the outermost shell of an atom. To determine the total number of valence electrons in H2O, add up the valence electrons of each atom. In H2O, the oxygen atom is more electronegative than hydrogen, so it will be the central atom. The hydrogens will be the outer atoms.
Molecular geometry h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons.
Coupon code xxl nutrition
Geometry of Molecules. How many lone pairs and bond pairs does water molecule have? The angular distance between tetrahedral bonds is but the angular distance between the hydrogen bonds is In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. Login To View Results. In the case of an ammonium molecule there is still a larger angle. Geometrical Structure called Lewis Structure The structure of the water molecule, which is called the Lewis Structure, determines that there are in total 8 valence electrons that help form the bonds in the triatomic molecule of water. H 2 O has a tetrahedral arrangement of molecules or an angular geometry. Feb 5, Water H2O molecule maintains neutrality. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. The central atom here is oxygen which is hybridized. Ans : Like every other molecule, water also has a molecular geometry. As the repulsion forces from the lone pairs are more than the repulsive forces of bonded pairs, the arrangement of atoms is distorted. The angle formed in the middle of two bonds of an atom is called a bond angle. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. Water H2O molecule maintains neutrality. Your result is as below. What is the meaning of Dihydrogen Monoxide molecule? Watch Now. What are the electron geometry and the molecular geometry of water? Both Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure.

0 thoughts on “Molecular geometry h2o”