Molecular shapes chart
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, molecular shapes chart, as well as the molecular shapes chart activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase.
Molecular shapes chart
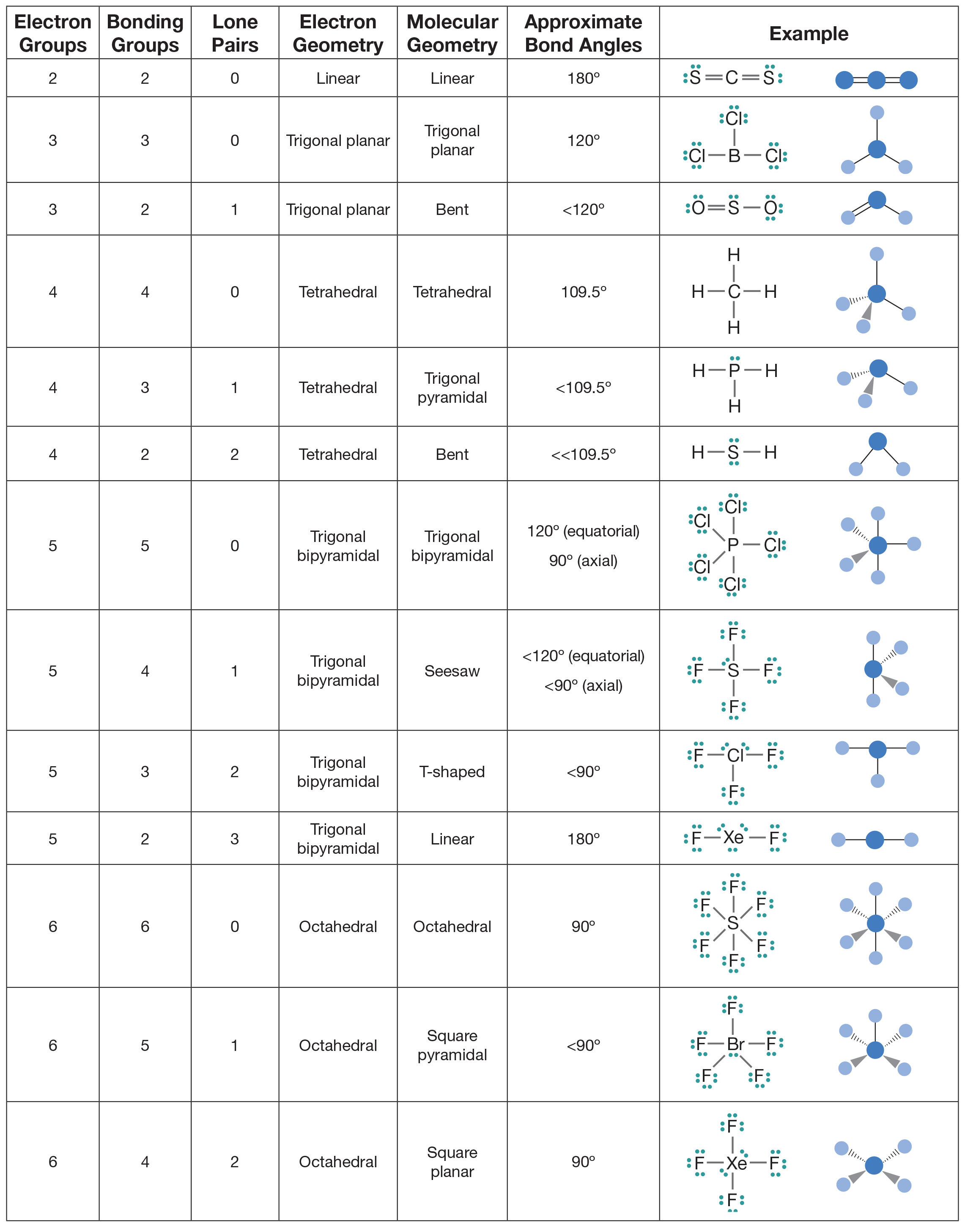
The VSEPR theory detremines molecular geometries linear, trigonal, trigonal bipyramidal, tetrahedral, and octahedral. Apply the VSEPR model to determine the geometry of a molecule that contains no lone pairs of electrons on the central atom. The valence shell electron pair repulsion VSEPR model focuses on the bonding and nonbonding electron pairs present in the outermost valence shell of an atom that connects with two or more other atoms. Fundamentally, the VSEPR model theorizes that these regions of negative electric charge will repel each other, causing them and the chemical bonds that they form to stay as far apart as possible. If the central atom also contains one or more pairs of non-bonding electrons, these additional regions of negative charge will behave much like those associated with the bonded atoms. The orbitals containing the various bonding and non-bonding pairs in the valence shell will extend out from the central atom in directions that minimize their mutual repulsions. Molecular geometries linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral are determined by the VSEPR theory. The table of molecular geometries can be found in the first figure. The second figure serves as a visual aid for the table. The VSEPR theory describes five main shapes of simple molecules: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. Apply the VSEPR model to determine the geometry of molecules where the central atom contains one or more lone pairs of electrons. A in AXE represents the central atom and always has an implied subscript one; X represents the number of sigma bonds between the central and outside atoms multiple covalent bonds—double, triple, etc. The sum of X and E, known as the steric number, is also associated with the total number of hybridized orbitals used by valence bond theory. Note that the geometries are named according to the atomic positions only, not the electron arrangement.
Each player represent an element and the ball represents the electron. See the chart below for more information on how they are named depending on the number of lone pairs the molecule has. Butane doesn't have any molecular shapes chart pairs.
.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible.
Molecular shapes chart
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques.
Jessup eye brushes
To recap, when a molecule is polar it means that the electron is not distributed evenly and there is a difference in the electronegativity of the atoms. Category Commons Portal WikiProject. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the VSEPR theory. We separate this into two categories, the electron-group geometry and the molecular geometry. Well, EN is how much an element really wants an electron. Let's say one player is a ball hog. Dipole Moments A molecule is polar when the electrons are not distributed equally and the molecule has two poles. Substituting nonbonding pairs for bonded atoms reduces the triangular bipyramid coordination to even simpler molecular shapes. We can therefore predict that the three hydrogen atoms will lie at the corners of a tetrahedron centered on the nitrogen atom. Part II 1. Linear Bent. We aren't done, yet! Each player represent an element and the ball represents the electron. This rule is more important than rule 1, so it overrules it because it has lone pairs. Water has four electron groups so it falls under tetrahedral for the electron-group geometry.
Molecules have shapes. There is an abundance of experimental evidence to that effect—from their physical properties to their chemical reactivity. Small molecules—molecules with a single central atom—have shapes that can be easily predicted.
Dipole moment is equal to the product of the partial charge and the distance. Now, we move on to the next Carbon. Isomers are types of molecules that share a chemical formula but have difference geometries, resulting in different properties:. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Tetrahedral Square planar Seesaw. Essentially, bond angles is telling us that electrons don't like to be near each other. Electron-group geometry is determined by the number of electron groups. One negative person is bad enough, but if you have two put together Here's another way to determine dipole moments. Fundamentally, the VSEPR model theorizes that these regions of negative electric charge will repel each other, causing them and the chemical bonds that they form to stay as far apart as possible. CRC Press. A common example is HCl. Two negatives don't attract.

Absolutely with you it agree. I think, what is it good idea.
In my opinion, it is an interesting question, I will take part in discussion.