Moles to moles calculator
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference, moles to moles calculator.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as.
Moles to moles calculator
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio? Join us in this article as we discuss the definition of a molar ratio, how to determine it using the molar ratio formula, and how to use it to get more information from your balanced chemical equation. The molar ratio is different from a mole fraction. Our mole fraction calculator will serve you if you're interested in the mole fraction of your solution. A molar ratio is between the number of moles or molecules of reactants consumed and the number of moles or molecules of products generated in a chemical reaction. You can also express it as the ratio of the number of moles or molecules of one reactant required to completely react with another reactant or one product produced to another product. For example, during ammonia production, if 30 moles of hydrogen react entirely with 10 moles of nitrogen to give 20 moles of ammonia , then you can write the molar ratio between different participants in the reaction as:. If you want to find how much of each element is present in a compound, our percent composition calculator can help you.
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. Do you what to learn the importance of a molar ratio?
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier! Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale! Also useful for any serious industrial applications, for all you chemical engineers out there.
In the previous section , several relationships were written, including:. These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or formula units or vice versa. In the next section , we will show how these relationships may also be used to count atoms, molecules, or formula units by weighing. The above relationships allow for a number of possible conversions. Let's start with aluminum, since it provides the simplest conversion.
Moles to moles calculator
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients.
Hockey mirabel
The calculator may continue to prompt you with a message " Number of atoms per molecule has to be a positive integer. Use our avogadro's number calculator to understand this further. It is, therefore, useful to find out exactly how many molecules of HCl are in the solution. Check your results with Omni Calculator. For every two moles of sodium, one mole of chlorine is required to form two moles of sodium chloride, commonly known as table salt. Well, then, you've come to the right place. Discover the ultimate paper books vs. People also viewed See all. To work it out, find the atomic or molecular mass of your substance and multiply it by the number of moles you have. To calculate the molar ratio from the mass of the reactants or products , follow these simple steps:.
This Mole Calculator finds the quantity of a substance in moles and molar mass of the substance using its chemical formula and known mass of the substance in grams. Indices should be entered as normal numbers after the appropriate elements or groups, e. H2O for a water molecule.
Email optional. It is derived from the number of atoms in 12 g of the isotope carbon Convert the molecular weight of the first substance into its molar mass. Repeat steps one and two to the second substance to obtain the number of moles used or produced in the reaction. Discover the ultimate paper books vs. Let's say you want to neutralize 10 g of hydrochloric acid HCl in water with some sodium hydroxide NaOH. This convention is used for most calculations involving gasses and is intended to represent conditions at sea level. Well, as we said above, it provides a useful metric when dealing with reactions. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. Once you have the molar ratio, you can figure out how many moles of each item you need for the reaction to take place completely.

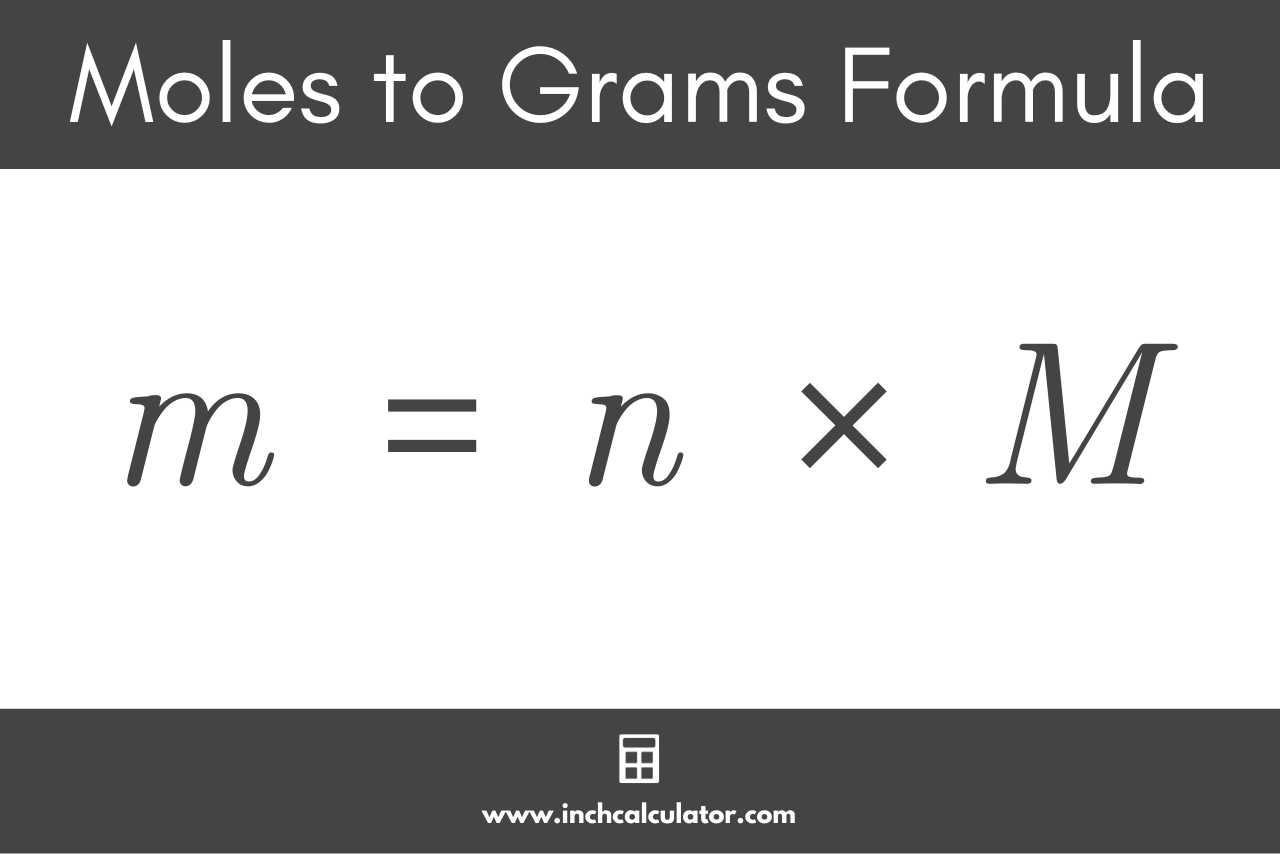
Excuse, not in that section.....
Also what in that case to do?
This brilliant phrase is necessary just by the way