N2o lewis dot
Skip to main content. Table of contents.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure.
N2o lewis dot
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am. Laurence Lavelle Skip to content. Quick links. Email Link. Re: Lewis Structure for N2O Post by chaggard » Mon Nov 05, am N goes in the center because you want to have the lowest ionization energy element in the center. The structure has a triple bond to the other N atom, and a single on the O atom.
For structures of larger molecules, in which more than one atom is joined to n2o lewis dot or more atoms, there is no longer a unique central atom, and the probability of encountering isomers dramatically increases. Gases 3h 54m. Enthalpy of Formation.
Cronk Syllabus Topics. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot structures. These symbolic representations were introduced by Gilbert Newton Lewis , a prolific American chemist who was a pioneer in the theory of chemical bonding, thermodynamics, and other areas of chemistry. Lewis structures are of great utility as a tool that allows us to speak a "language" of chemical bonding and molecular structure. The structures, with their guiding rules principally the octet rule provide a basis to predict the chemistry of the elements. In other words, we can make a judgment of whether a particular combination of elements is likely to form a stable molecule or polyatomic ion.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 0. The central N makes one covalent bond with N and O. The molecule is neutral but in resonance, its show different canonical form, and some of them are charged.
N2o lewis dot
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well.
Ottawa weather 7 days
Formal Charge. Double and triple bonds are allowed. The Electron Configuration Review. For a molecule uncharged , that count is the correct number of valence electrons. The Ideal Gas Law: Density. Dipole Moment. Resonance Structures and Formal Charge. See the Big List of Lewis Structures. Naming Esters. Arrhenius Equation. Nitrogen has a higher ionization energy because it has a half-filled 2p shell that makes it more stable unlike oxygen which has 2 unpaired electrons in its 2p shell which results in more electron repulsions and therefore lowers the ionization energy.
But have we ever tried to know more about this gas that can make humans laugh? I guess no!
Pressure Units. Intro to Crystal Field Theory. Neutron to Proton Ratio. Internal Energy. Law of Multiple Proportions. Finally, the remaining hydrogen atom attaches to one of the oxygen atoms. Van der Waals Equation. Subtract this number from the number of valence electrons for the neutral atom:. Heating and Cooling Curves. Formal charge is only a useful bookkeeping procedure; it does not indicate the presence of actual charges. There are 16 valence electrons in N 2 O. Learning objectives and questions Draw valid Lewis structures for molecules and polyatomic ions Page updated References Tro NJ.

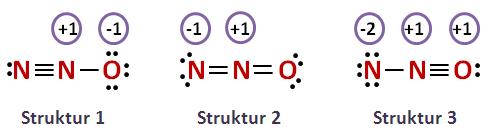
Between us speaking, I would try to solve this problem itself.