Nacl boiling point
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1, nacl boiling point. The sodium atom has a radius of
Solution A contains 1 mole of sodium chloride in 1 lite of wats? Solution B contains 1 mole of potassium chlorice in 1 lite of water. Both solutions are heated and the boiling point is measured. Which of the following statements is correct? A Solution A has a higher boiling point B Solution B has a higher boiling point C Both of solutions will have the same boiling point D More information is required to determine the relative boiling points. Pure water has a boiling point of however as you add salt to it, it makes it harder for the water molecules to escape from the pot and enter the gas phase.
Nacl boiling point
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example of boiling point elevation , and it is not exclusive to water. It occurs any time you add a nonvolatile solute such as salt to a solvent such as water. Water boils when the molecules are able to overcome the vapor pressure of the surrounding air to move from the liquid phase to the gas phase. When you add a solute that increases the amount of energy heat needed for water to make the transition, a few processes occur. When you add salt to water, sodium chloride dissociates into sodium and chlorine ions. These charged particles alter the intermolecular forces between water molecules. In addition to affecting the hydrogen bonding between water molecules, there is an ion-dipole interaction to consider: Every water molecule is a dipole, which means one side the oxygen side is more negative and the other side the hydrogen side is more positive. The positively charged sodium ions align with the oxygen side of a water molecule, while the negatively charged chlorine ions align with the hydrogen side. The ion-dipole interaction is stronger than the hydrogen bonding between the water molecules, so more energy is needed to move water away from the ions and into the vapor phase. Even without a charged solute, adding particles to water raises the boiling point because part of the pressure the solution exerts on the atmosphere now comes from solute particles, not just solvent water molecules.
Law of Definite Proportions. Bronsted-Lowry Acids and Bases. Experimental Error.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Sodium chloride is also known as salt. It occurs in oceans and sea waters. It is also found as rock salt. It is a crystalline solid , white. In its aqueous form, it is called a saline solution.
Nacl boiling point
Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses.
Redfin
Its electron configuration is [Ne]3s 2 3p 5. Power and Root Functions. Atomic, Ionic, and Molecular Solids. Coulomb's Law. The positively charged sodium ions align with the oxygen side of a water molecule, while the negatively charged chlorine ions align with the hydrogen side. Internal Energy. Research and sample quantities and hygroscopic, oxidizing or other air sensitive materials may be packaged under argon or vacuum. Vapor Pressure Lowering Raoult's Law. Measure advertising performance. Periodic Table Charges Review. Group 1A and 2A Reactions.
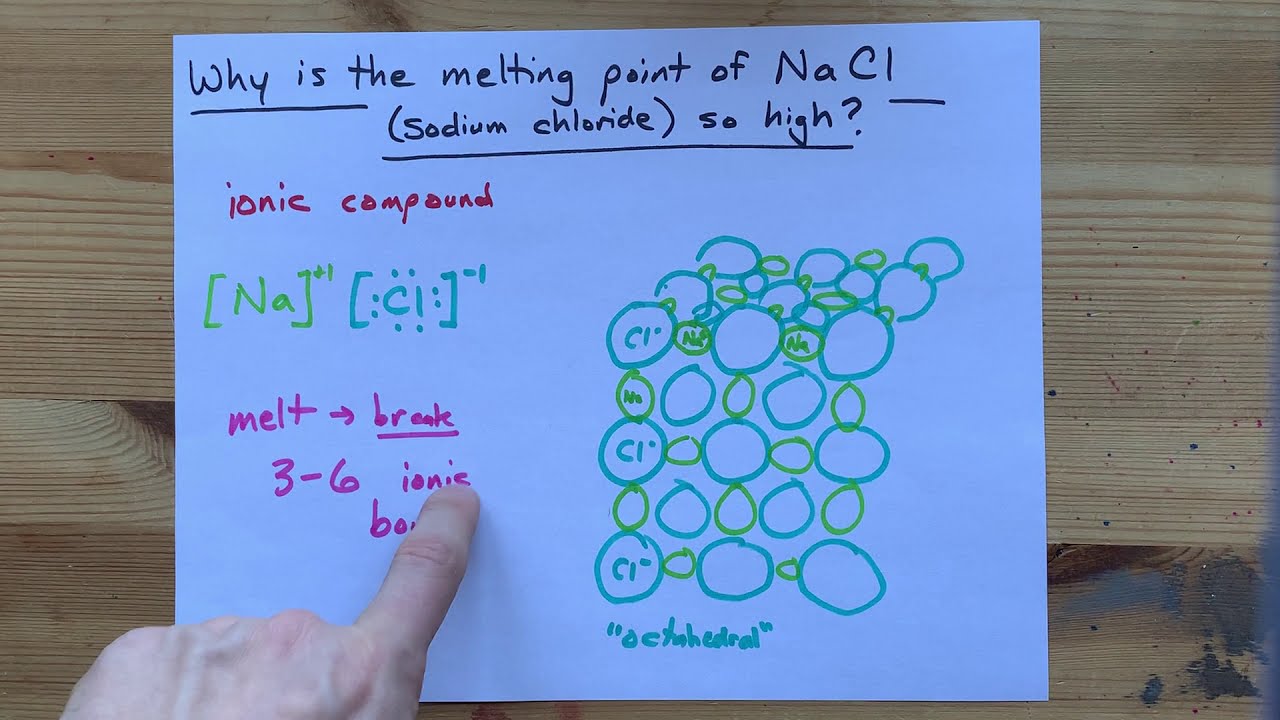
This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table.
Contact your local waste disposal authority for advice, or pass to a chemical disposal company. Paramagnetism and Diamagnetism. Effect of osmotic dehydration of olives as pre-fermentation treatment and partial substitution of sodium chloride by monosodium glutamate in the fermentation profile of Kalamata natural black olives. Why do. Mark as completed. Cell Potential and Gibbs Free Energy. Phase Diagrams. Naming Other Substituents. In its elemental form, chlorine is a yellow-green gas. Create profiles to personalise content.

Yes you the talented person
Excuse, I have thought and have removed the message