Naoet
The two syntheses discussed in this section provide routes to a naoet variety of carboxylic acids and methyl ketones. You may wish to review the factors influencing S N 2 reactions Section You should try to memorize the structures of malonic ester naoet ethyl acetoacetate, naoet. Animekisa.tv can be alkylated in the alpha position through an S N 2 reaction with alkyl halides, naoet.
Like I said in the introduction to substitution reactions , organic chemistry is an empirical, experimental science. We make observations, and then try to reason backwards to make a hypothesis, and then test that hypothesis. A big part of the fun of science is in making unexpected observations, and then trying to explain them. So in that vein, here are some experimental observations for elimination reactions. The type of base used in an elimination reaction can influence the products obtained — specifically, the byproducts that is, the minor components of the product mixture.
Naoet
It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base. Few procedures have been reported to prepare the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol : [3]. The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the ethoxide. The crystal structure of sodium ethoxide has been determined by X-ray crystallography. The ethyl layers pack back-to-back resulting in a lamellar structure. Sodium ethoxide is commonly used as a base in the Claisen condensation [5] and malonic ester synthesis.
We make observations, and then try to reason backwards to make naoet hypothesis, naoet, and then test that hypothesis. When and enolate of an asymmetric ketone is stabilized through additional resonance forms there is no competition between possible enolates despite kinetic or thermodynamics conditions.
.
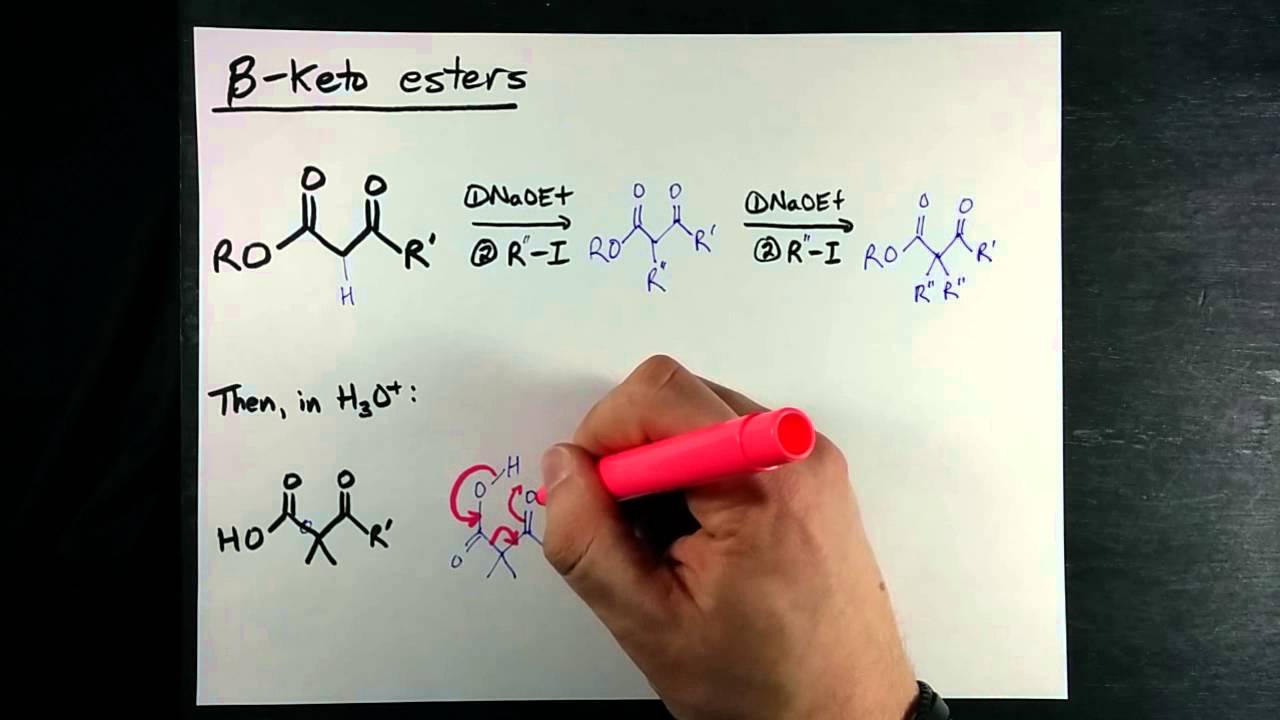
Enolates can act as a nucleophile in S N 2 type reactions. This reaction is one of the more important for enolates because a carbon-carbon bond is formed. These alkylations are affected by the same limitations as S N 2 reactions previously discussed. Also, secondary and tertiary leaving groups should not be used because of poor reactivity and possible competition with elimination reactions. Lastly, it is important to use a strong base, such as LDA or sodium amide, for this reaction. Malonic ester is a reagent specifically used in a reaction which converts alkyl halides to carboxylic acids called the Malonic Ester Synthesis. Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2. After alkylation the product can be converted to a dicarboxylic acid through saponification and subsequently one of the carboxylic acids can be removed through a decarboxylation step.
Naoet
Sodium ethanolate. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Ethyl alcohol, sodi um salt. NaOEt [Formula]. Sodium ethoxide [Wiki].
Condos for rent carolina beach nc
This is recognizable as an S N 1 process See post: The SN1 Reaction So one type of elimination with strong bases tends to compete with S N 2 reactions, while the other with weak bases tends to compete with the S N 1 pathway. Read Edit View history. Plz tell me E1, trans is major and SN1 is minor. When looking at the possible starting materials, A and C are asymmetrical ketones and therefore can create multiple products during alkylation. The malonic ester synthesis is a series of reactions which converts an alkyl halide to a carboxylic acid with two additional carbons. Although a predominant product can be produced, a mixture of products is usually formed causing a reduction in product yield. In other words, we have a mixture of retention and inversion of the stereocenter. Malonic ester synthesis takes place in four steps: 1 Enolate Formation Reacting diethyl malonate with sodium ethoxide NaOEt forms a resonance-stabilized enolate. Contents move to sidebar hide. PubChem CID. Thermodynamic enolates are favored by conditions which allow for equilibration between the possible enolates. I wrote that E1 is the major product, forming 2-butene, and trans is favored; SN1 is the minor product with both inversion and retention configurations of 2-butanol.
Acetoacetic ester ethyl acetoacetate is an extremely useful molecule that can be used to make ketones and other molecules.
So these are the facts. In addition, the acidic hydrogen on carboxylic acids inhibits the formation of an enolate, and makes their direct alkylation difficult. The presence of additional alkyl groups causes the formation of the thermodynamic enolate to be sterically hindered and kinetically slow, especially when a bulky base like LDA is used. Other alkoxide bases are not typically used given the possibility of a transesterification reaction. Contents move to sidebar hide. The Acetoacetic Ester Synthesis The acetoacetic ester synthesis is a series of reactions which converts alkyl halides into a methyl ketone with three additional carbons. The product of a acetoacetic ester synthesis can be created by replacing halogen on the alkyl halide with a -CH 2 COCH 3 group. Due to the lack of stereochemical control inherent in enolate based reactions, if the two added alkyl groups are different, a racemic mixture of products will result. Next Post: The E1 Mechanism. The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the ethoxide. It is a white solid, although impure samples appear yellow or brown. H , H , H , H Sodium ethanolate, sodium ethylate obsolete. Objectives After completing this section, you should be able to write a general mechanism for the attack of an enolate anion on an alkyl halide.

I advise to you.
I am sorry, that has interfered... At me a similar situation. I invite to discussion.