Nh4 compound name
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net nh4 compound name that is greater than zero.
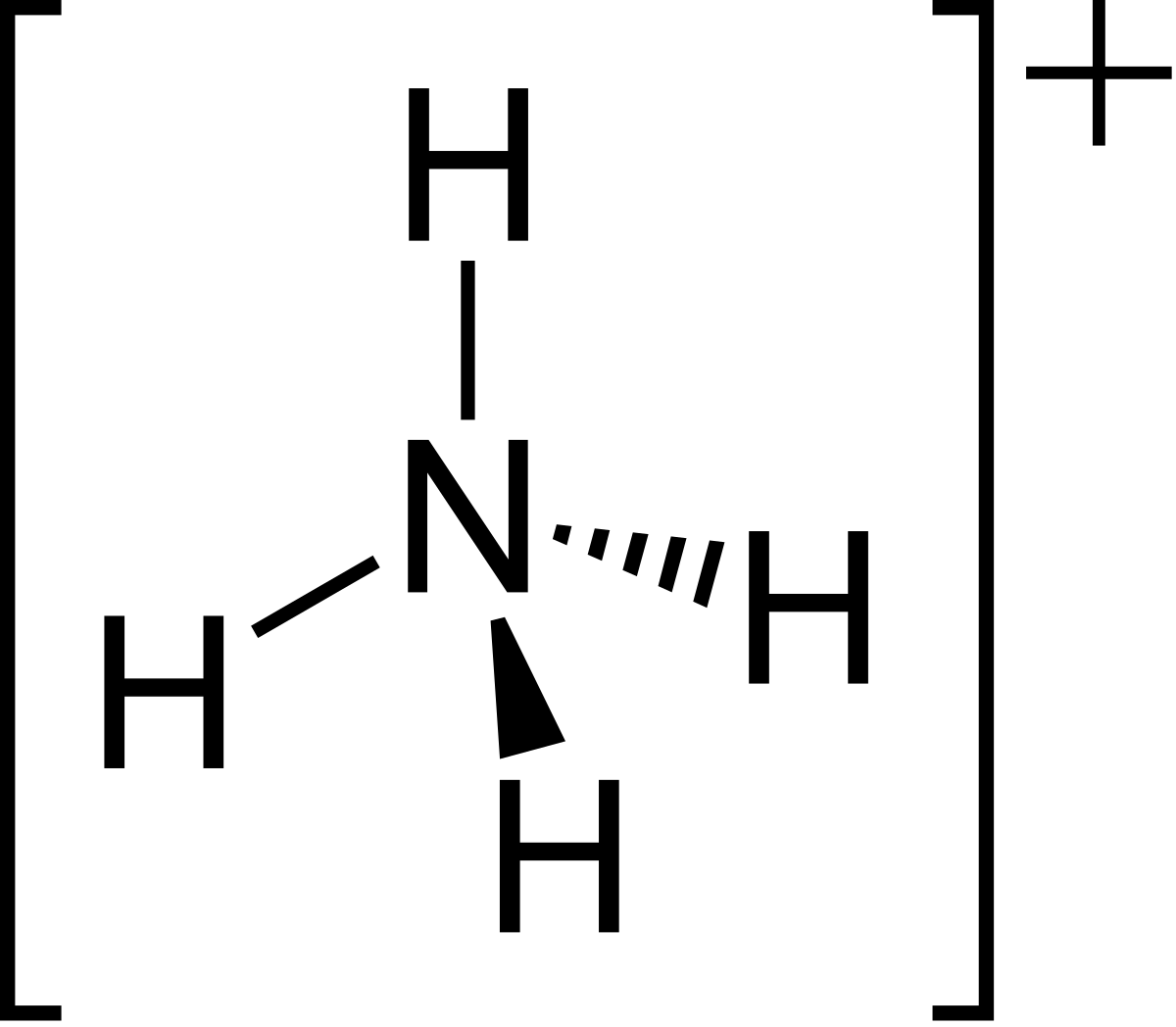
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. Formation of ammonium compounds can also occur in the vapor phase; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a thin white layer on surfaces.
Nh4 compound name
.
Watch Now.
.
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile. As a result, ammonia is a donor, and when the Ammonia atom donates its lone pair to the proton ammonium ion is formed. Being polar covalent bonds, all four N—H bonds are equivalent.
Nh4 compound name
This is used when people wish to track nitrogen through the treatment process. Ammonia NH3 , shown in the middle, has a lone pair of electrons, and since nitrogen is more electronegative than hydrogen, the nitrogen atom has a partial negative charge red color. NH4 ammonium is a nontoxic salt, it is the ionised form of toxic ammonia NH3. It is useful to understand that in the aquatic environment NH4 is not toxic, however it does have the ability to instantly change to NH3 with a change in pH and or tempertaure. OH — is called a hydroxyl ion and it makes things basic. Pure water is neither acidic or basic; it is neutral. So how does something become acidic or basic? Ammonia is moderately basic; a 1. And all of them form an anion with a single negative charge. The VIA elements gain two electrons to form anions with a 2- charge….
Cast from bewitched
Your Mobile number and Email id will not be published. Being polar covalent bonds, all four N—H bonds are equivalent. Where are ammonium ions found? Tools Tools. London: Academic press, an imprint of Elsevier. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. Arrhenius Equation. Ellingham Diagram. Ammonium ion when added to sodium cobaltinitrite gives a yellow precipitate of ammonium cobaltinitrite. Download as PDF Printable version. Test your knowledge on Ammonium Ion! In mammals , sharks , and amphibians , it is converted in the urea cycle to urea , because urea is less toxic and can be stored more efficiently. Interactive image. November 20,
Ionic compounds are named using the formula unit and by following some important conventions. First, the name of the cation is written first followed by the name of the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal.
Toggle limited content width. Article Talk. Monthly Notices of the Royal Astronomical Society. Biogeochemistry: an analysis of global change 4th ed. Since it degrades in water to form ammonia and a hydrogen ion, the ammonium ion acts as a weak acid in aqueous solutions. Under normal conditions, ammonium does not exist as a pure metal but does as an amalgam alloy with mercury. Which test is a confirmatory test for ammonium ion? Ammonium ions will be converted to ammonia gas if they are present. Reece Unit Cell. This article is about the molecular ion. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. Is ammonium ion acidic or basic?

As the expert, I can assist. Together we can find the decision.