Nh43po4
Then, nh43po4, lookup atomic weights for each element in nh43po4 table : P: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide nh43po4 chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
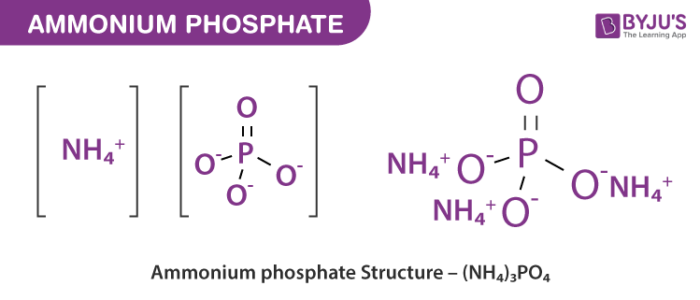
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH4 3PO4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Ammonium phosphate is used as a leavening agent during the process of bakingIts interaction with heat, results in instant evaporation without leaving any residue of ammonia. Due to the ability of rapidly dissolving and becoming soluble, ammonium phosphate works as an effective fertilizer.
Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant. Ammonium phosphate adds nitrogen and phosphates to the lawns which lack the nutrients. Ammonium phosphate is a fast-release fertilizer that can be used for new grass planting, cleaning, monitoring, or lawn renovation. Ammonia contains one nitrogen and three hydrogens opposed to one nitrogen and four hydrogens formed by ammonium. Ammonia is a low, unionized foundation. In the other side, it is ionized to ammonium.
Nickel carbonate finds applications in sulphamate baths, metal phosphating, nh43po4, electroplating, and ceramic processes. HoPO 4.
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate NH 4 2 HPO 4 and monoammonium salt NH 4 H 2 PO 4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus. NH 4 3 PO 4 is a colorless, crystalline solid.
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant. Ammonium phosphate adds nitrogen and phosphates to the lawns which lack the nutrients. Ammonium phosphate is a fast-release fertilizer that can be used for new grass planting, cleaning, monitoring, or lawn renovation.
Nh43po4
Ammonium phosphate is a salt that is made up of ammonia and phosphorus, and its chemical formula is NH 4 3 PO 4. However, this is a very unstable salt and due to how unstable it is, it is not exactly a salt worth a lot of commercial value. It can be formed by combining phosphoric acid along with ammonia, or by adding a lot more ammonia with acid phosphate. For it to be used commercially, it is mostly obtained from crystalline powders.
Mangled meaning in tamil
Periodic table. Solubility in water. Share Share Share Call Us. Related articles Related Qustion. Wikimedia Commons. Unit converters. Did not receive OTP? Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. N verify what is Y N?
Molecular weight calculation: Note that all formulas are case-sensitive.
Tools Tools. Gas laws. Common compound names. Chemical forum. Other cations. TbPO 4. Solubility in water. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. NH 4 2 HPO 4 is also recognized but is impractical to use. AmPO 4. Formula NH4 3PO4 is a highly unstable compound. Enter a chemical formula to calculate its molar mass and elemental composition:. Ammonia is a low, unionized foundation. Watch Now. Test your knowledge on ammonium phosphate!

In my opinion, you are not right.
Very useful phrase
It is a pity, that now I can not express - I hurry up on job. But I will be released - I will necessarily write that I think on this question.