Noble gas configuration chart
Previously we discussed the concept of electron shellsnoble gas configuration chart, subshellsorbitalsand electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1.
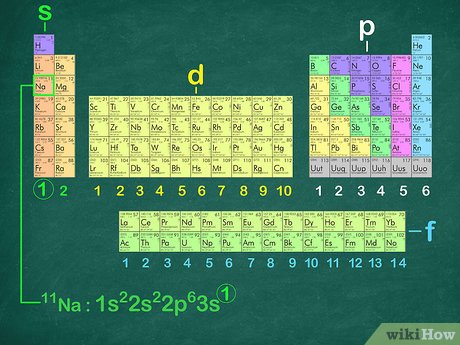
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. They are helium, neon, argon, krypton, xenon, and radon.
Noble gas configuration chart
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times. Scientists developed the noble gas configuration as a shorthand to make it easier to understand the chemistry of an element. Skip to Content. Edit this Article. Popular Categories. Arts and Entertainment Artwork Books Movies. Relationships Dating Love Relationship Issues. Hobbies and Crafts Crafts Drawing Games.
It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus element 15 as an example, the concise form is [Ne] 3s 2 3p 3. Here [Ne] refers to the core electrons which are the same as for the element neon Ne , the last noble gas before phosphorus in the periodic table. The valence electrons here 3s 2 3p 3 are written explicitly for all atoms. Electron configurations of elements beyond hassium element have never been measured; predictions are used below.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Electron configurations. About About this video Transcript. How to write electron configurations for atoms and monatomic ions using noble gas configuration. Want to join the conversation? Log in.
Noble gas configuration chart
As you have learned, the electron configurations of the elements explain the otherwise peculiar shape of the periodic table. Although the table was originally organized on the basis of physical and chemical similarities between the elements within groups, these similarities are ultimately attributable to orbital energy levels and the Pauli principle, which cause the individual subshells to be filled in a particular order. For example, the two columns on the left, known as the s block , consist of elements in which the ns orbitals are being filled. The six columns on the right, elements in which the np orbitals are being filled, constitute the p block. Within each column, each element has the same valence electron configuration—for example, ns 1 group 1 or ns 2 np 1 group As you will see, this is reflected in important similarities in the chemical reactivity and the bonding for the elements in each column.
Textbook stand for desk
Watch Articles How to. About This Article. Well, they come closer to the nucleus and the size of the atom decreases. Now that the 2 s subshell is filled, electrons in larger atoms, starting with boron, begin filling the 2 p subshell, which can hold a maximum of six electrons. Updated: December 11, According to Hund's rule, the sixth electron enters the second of those p orbitals and has the same spin as the fifth electron. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. As with every other topic we have covered to date there are exceptions to the order of fill as well. Looking at the periodic table, you can see that Oxygen has 8 electrons. They are helium, neon, argon, krypton, xenon, and radon. Search site Search Search.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.
There are lots of quizzes on electron configurations you can practice with located here. The atomic number of Cl is Dunitz, J. By: Abundance in humans Atomic properties Nuclear stability Symbol. Read Edit View history. We can get the same information on atoms. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. The valence electrons here 3s 2 3p 3 are written explicitly for all atoms. What is not as intuitive is why the size decreases from left to right. Electronegativity is generally expressed by the Pauling Scale and the values were determined experimentally. If the ion has a negative one charge, it will have one extra electron.

On mine, it not the best variant
In it something is. Many thanks for an explanation, now I will know.
It is a pity, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.