Pcl3 electron domain geometry
When we talk about the hybridization of PCl 3 students should not confuse it with PCl5. PCl 3 is sp 3 hybridized.
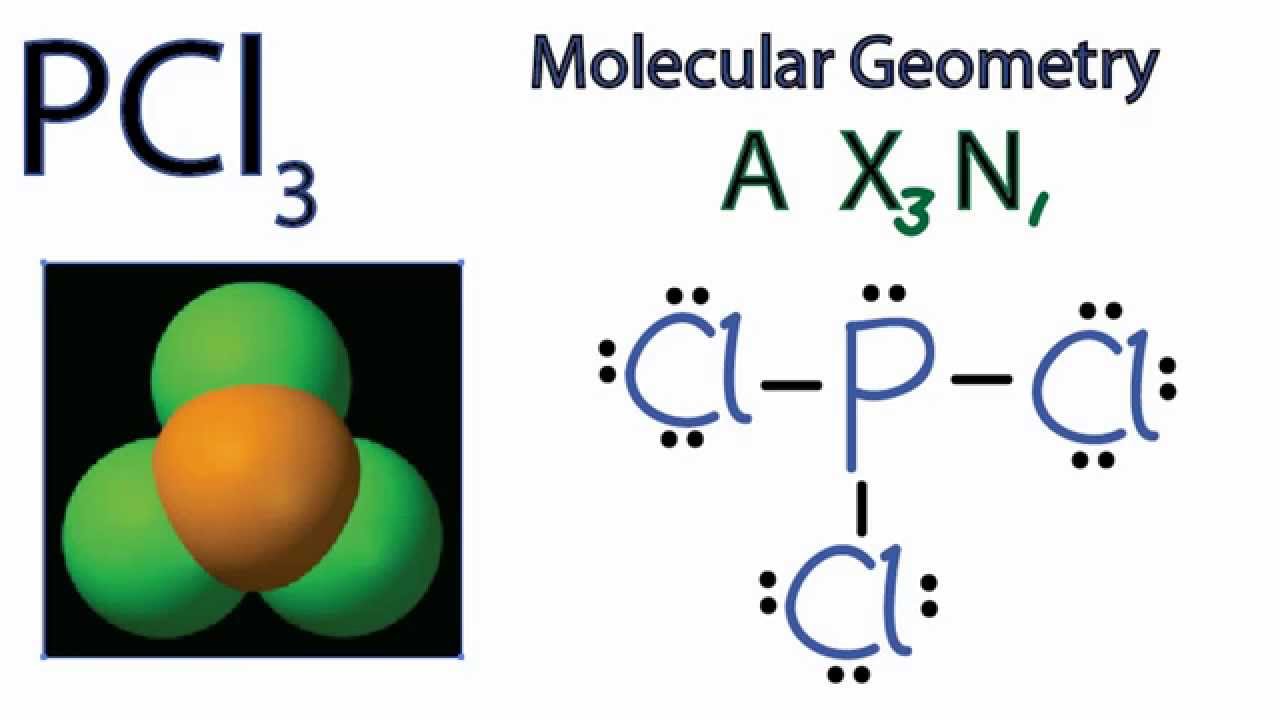
The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl. The PCl3 Lewis structure is shown below:. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively.
Pcl3 electron domain geometry
.
View Result. Let us look at how this hybridization occurs and we will also understand the molecular geometry of this compound.
.
Total of 26 valence electrons are utilized. Electronegativity difference: P 2. Drawing the Lewis structure for PCl3 phosphorus trichloride involves a series of steps to understand its molecular composition and bonding. Count the Valence Electrons : Phosphorus P is in Group 15 of the periodic table, so it has 5 valence electrons. Chlorine Cl is in Group 17, with 7 valence electrons. Then, draw single bonds connecting the phosphorus atom to each chlorine atom.
Pcl3 electron domain geometry
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them.
Factoring polynomials activity pdf
Mar 8, In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. XeF2 Lewis structure: drawing, hybridisation, geometry. One of the hybrid orbitals will contain one lone pair of electrons. PCl3 Hybridisation Phosphorus in PCl3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. Post My Comment. These hybrid orbitals overlap with the p orbitals of chlorine atoms, creating sigma bonds and maintaining the trigonal pyramidal molecular shape of PCl3. Order: 1bag Purity: 99 Supply Ability: There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl.
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.
Step 4 Stability of structure In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. Step 2 Identify the central atom The central atom must be highly or minimally electronegative. Phosphorus trichloride manufacturers. PCl3 Hybridisation Phosphorus in PCl3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. Does it have to be refrigerated? Post My Comment. Further, each chlorine atom also holds 3 lone pairs. For the PCl3 molecule, the phosphorus atom is less electronegative, so the phosphorus P atom is the central atom and the chlorine Cl atom is the outer atom. XeF2 Lewis structure: drawing, hybridisation, geometry. Login To View Results. Your result is as below. Did not receive OTP? Your Mobile number and Email id will not be published. Download Now.

Bravo, seems brilliant idea to me is
Rather amusing piece
It agree, a useful piece