Sf4 bond angle
What is the shape of SF 4 including bond angles? The formula used to calculate the hybridization of a molecule is as follows:. V is the number of valence electrons present sf4 bond angle the central atom. N is the number of monovalent atoms bonded to the central atom.
The process of mixing of atomic orbitals belonging to the same atom of slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new sets of orbitals of equivalent energies and shape is called hybridization. The new orbitals in this form are known as hybrid orbitals. Like pure orbitals the hybrid orbitals are used in Bond formation. Hybridization is a hypothetical concept and has been introduced in order to explain the characteristic geometrical shapes of polyatomic molecules. The central atom is S. So, to explain in simple terms, its bonding regions are four having one lone pair.
Sf4 bond angle
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. In addition, two electrons will be kept as lone pair in the sulphur atom. When bonding takes place there is a formation of 4 single bonds in sulphur and it has 1 lone pair. Looking at this, we can say that the number of regions of electron density is 5.
Sulphur will use five orbitals: one 3s orbital, three 3p orbitals, sf4 bond angle, and one 3d orbital. JEE Examination Scheme. So, to explain in simple terms, its bonding regions are four having one lone pair.
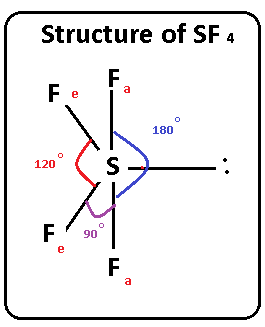
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions.
Sf4 bond angle
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure , there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs and bonding pairs of electrons. The SF4 molecule has a seesaw shape , with the sulfur atom at the center and the fluorine atoms surrounding it.
Gerry jacket
Because 3s orbitals in sulphur are entirely filled but 3p orbitals in 4f are not, 4 half-filled orbitals, or orbitals with just one electron in each orbital, are required to form bonds. Let us learn about the SF4 molecular geometry and bond angles. There is substantially less repulsion when the angle is extended by analysing degrees. Melting Point. Share Share Share Call Us. Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see not bonded. See all questions in Molecular Geometry. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Sulphur tetrafluoride is made up of only two elements: sulphur and fluorine. JEE Coaching Centres. This is a polar covalent bond because it shows an uneven sharing of electrons. As a result, SF4 is polar. Fluorine is a periodic table group VIIA element with seven electrons in its final shell. Challenge Yourself Everyday.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
Lines represent bonds created between two atoms, whereas dots represent valence electrons that do not make any bonds. There are four of the hybrid orbitals overlapped with 2P-orbitals. This article is being improved by another user right now. Sulfur will use 5 orbitals, including 1 3s-orbital, 3 3p-orbitals, and 1 3d-orbital. What is the shape of the SeF 4 molecule? JEE Coaching Centres. Four Flourine atoms bond with sulfur, thus out of six, four electron got bonded and a pair of electron is unbonded which is called lone pair of electron. What is SF4's molecular geometry? Put your understanding of this concept to test by answering a few MCQs. In SF 4 , a nonbonding sulphur lone electron pair replaces one of these ligands, while the rest are fluorines. Besides, the 4 fluorine atoms will have 3 lone pairs of electrons in their octet, which will utilize 24 valence electrons further. Occurs due to lone pair interactions. Enhance the article with your expertise. Sulphur Tetrafluoride contains 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the core atom in its Lewis structure. Let us learn about the SF4 molecular geometry and bond angles.

I think, that anything serious.
I consider, that you are mistaken. Let's discuss. Write to me in PM, we will communicate.
I confirm. It was and with me. We can communicate on this theme.