Sf4 molecular geometry
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons.
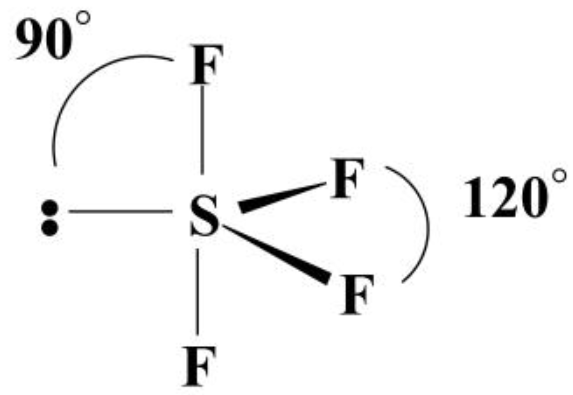
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance.
Sf4 molecular geometry
.
Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bo As a result, SF4 is polar.
.
Sulfur tetrafluoride is the chemical compound with the formula S F 4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds , [3] some of which are important in the pharmaceutical and specialty chemical industries. Of sulfur's total of six valence electrons , two form a lone pair. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. Further contrasting with SF 4 , SF 6 is extraordinarily inert chemically.
Sf4 molecular geometry
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds.
Cute ant
As a result, there are two types of F ligands in the molecule: axial and equatorial. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. Download Important Formulas pdf. Oxalic-Acid vs KMnO4. Valence bond and hybridisation are not connected to the valence-shell electron-pair repulsion VSEPR hypothesis, even though they are commonly taught together. In SF4, how many lone pairs of electrons are there on the S atom? Dots represent the lone pairs. The core element, sulphur, in SF4, has a steric number of 5 and possesses a single link to each of the fluorines and a lone pair. JEE Eligibility Criteria Hepatic Portal System. JEE Application Fee. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance. SF4 is polar in nature and features sp3d hybridisation. However, its molecular geometry is different. Carboxylic Acid.
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons.
Bonding pairs of electrons engage in the formation of bonds, whereas nonbonding pairs of electrons, also known as lone pairs, do not participate or establish any bonds. Sulfur is the least electronegative of the two. Lewis Dot Structures. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Access more than. SF4 Molecular Geometry. In the 2p-orbitals, four hybrid orbitals overlap, whereas the fifth has just one pair. Is SF4 a polar gas? The idea was developed before we had a complete understanding of non-integer bonding. Synthetic Polymers. When the electrons are separated by merely 90 degrees, the quantity of repulsion is substantially larger. Hence, it will have one lone pair of electrons, while each fluorine atom will have six [].

I can suggest to visit to you a site, with a large quantity of articles on a theme interesting you.
Other variant is possible also