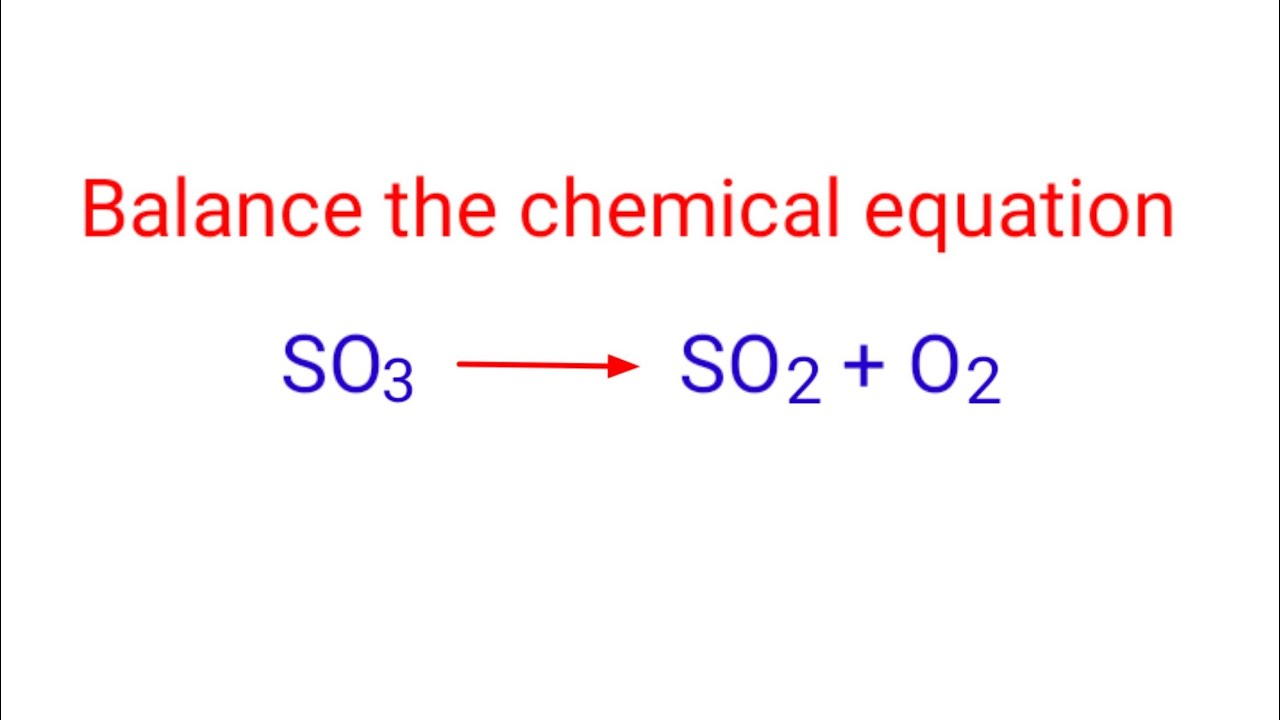
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O"and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have so2 o2 so3 balanced S" on the right, so we need "1 S" on the left.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
So2 o2 so3 balanced
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors. Dimensional Analysis.
Periodic Trend: Successive Ionization Energies. Let's balance this equation using the inspection method. Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'.
.
The coefficients show the number of particles atoms or molecules , and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged — this follows from the law of conservation of mass of substances. This is an oxidation-reduction redox reaction. S O 2 is a reducing agent, O 2 is an oxidizing agent.
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26, Explanation: You follow a systematic procedure to balance the equation. Another useful procedure is to start with what looks like the most complicated formula.
5 letter words starting with s
It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Hydrogenation Reactions. How can I balance this chemical equation? Another useful procedure is to start with what looks like the most complicated formula. You can reuse this answer Creative Commons License. Addition and Subtraction Operations. Molecular Polarity. Naming Coordination Compounds. Paramagnetism and Diamagnetism. Rutherford Gold Foil Experiment. Intro to Electrochemical Cells. Radioactive Half-Life. If you do not know what products are, enter reagents only and click 'Balance'. Transition Metals and Coordination Compounds 3h 14m.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction.
Le Chatelier's Principle. Strong-Field vs Weak-Field Ligands. Gamma Emission. Table of contents. Energy Diagrams. Collision Theory. Acid and Base Equilibrium 5h 4m. Molecular Equations. Periodic Table Charges Review. Molecular Formula. Subatomic Particles. Another useful procedure is to start with what looks like the most complicated formula. Cell Potential and Equilibrium. Best for: Equations that are more complex and not easily balanced by inspection. Combustion Analysis.

0 thoughts on “So2 o2 so3 balanced”