So4 2 formal charge
Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl. A: Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a…. Q: How many of the following statements are true? For each…. A: In methyl azide three nitrogen atoms make covalent bond with carbon and with nitrogen. The electrons….
So4 2 formal charge
What is the formal charge on Cl in the following lewis structure. Calculate the formal charge on Cl atom in H C l O 4. Octet is completed in which of the following? Assuming Lewis Octet the What will be the bond pair and lone pair ratio in the given structure Which of the following has linear in shape? Which of the molecule has p-p overlapping? The hybridisation 'S' in SO 2 is. For 5 s electron the values of n,l,m,s respectively could be. Which of the following is correct for H-F bond length? The ratio of number of sigma -bond to pi- bond in N 2 and CO 2 molec
Opens New Window. Pirani, S.
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it. This satisfies the octet rule allowing nitrogen's valence shell of electrons to look just like the noble gas neon's.
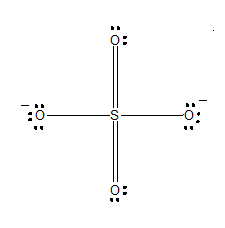
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more. In this blog post, we will go through all the details related to this molecule. Right from valence electrons to shape, you will find everything related to SO ion here. For determining the Lewis Structure for any molecule, we first need to know the total number of valence electrons.
So4 2 formal charge
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Dot structures and molecular geometry. About About this video Transcript. Definition of formal charge, and how minimization of formal charge can help choose the more stable dot structure. Created by Jay. Want to join the conversation? Log in.
Walmart hours jan 1 2024
Despite the cases for expanded octets, as mentioned for incomplete octets, it is important to keep in mind that, in general, the octet rule applies. Naming Alkynes. Trigonal bi pyramidal geometry have Resonance Structures of NO3 -1 , nitrate ion. Renard, J. Place remaining electrons on the central atom, usually in pairs. Connect the atoms with a pair of electrons in each bond. A: Formal charge is the charge on an atom present in a molecule assuming no effect of electronegativity…. Cell Potential and Equilibrium. A: Lewis structure: Lewis structures are diagrams that show the bonding between atoms of a molecule and…. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. A: The Lewis structures of the SO are as follows:. Gomis, M. And the bonding electrons, 2, 4, 6, 8; we've used 8 of those, and we'll divide that by 2.
The SO4 2- Sulfate Ion , comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties. At the heart of comprehending the characteristics and reactivity of SO4 2- lies the exploration of its Lewis structure. Determine Total Valence Electrons.
Intermolecular Forces and Physical Properties. Reaction Quotient. Intro to Acid-Base Titration Curves. No formal charge at all is the most ideal situation. Strong Titrate-Strong Titrant Curves. Cell Potential: Standard. A: In methyl azide three nitrogen atoms make covalent bond with carbon and with nitrogen. Sulfur and oxygen are in the same group but sulfur is below oxygen so it will most likely be the central atom in the structure:. First Law of Thermodynamics -. Chemistry for Engineering Students. As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent.

Curiously....
Now all is clear, thanks for an explanation.
I am final, I am sorry, but it does not approach me. There are other variants?