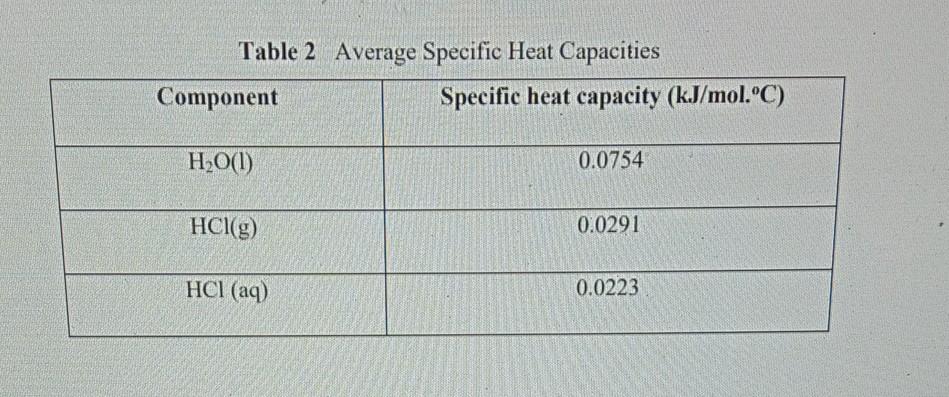
Specific heat of hcl
Login to see your most recently viewed materials here. Or if you don't have an account with us yet, then click here to register. Hydrochloric acid is a strong acid used as chemical reagent, pH-regulating, ion exchanger regenerating, pickling or cleaning agent. To see MatWeb's complete data sheet for this material including material property data, metal compositions, material suppliers, specific heat of hcl, etcplease click the button below.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table.
Specific heat of hcl
.
Absolute intensity measurements Chamberlain and Gebbie,Sanderson, Tokuhiro, Tokuhiro, T. A, 66,
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:.
Specific heat of hcl
Water has a high capacity for absorbing heat. In a car radiator, it serves to keep the engine cooler than it would otherwise run. As the water circulates through the engine, it absorbs heat from the engine block. When it passes through the radiator, the cooling fan and the exposure to the outside environment allow the water to cool somewhat before it makes another passage through the engine. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. The heat that is either absorbed or released is measured in joules. The mass is measured in grams.
Little morocco corvallis
To obtain the physical solubility of HA, the value has to be divided by the acidity constant K A. Radiative Transfer , , 2, Meot-Ner Mautner and Sharon G. Howlett, Howlett, K. Leavitt, Baker, et al. Select a region with data to zoom. Larson and McMahon, Larson, J. Atwood, Vu, et al. Quantitative scales of lewis acidities from ICR halide exchange equilibria , J. Bartmess M - Michael M. Watson, Watson, J. Liebman, and Stephen E.
In equation form, this can be represented as the following:. That is if a constant has units, the variables must fit together in an equation that results in the same units.
Moselhy and Pritchard, Moselhy, G. Alamichel and Legay, Alamichel, C. MatWeb is intended for personal, non-commercial use. Kaiser, Kaiser, E. Leningrad , , 42, In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:. Rodova, Shevtsova, et al. Herman and Asgharian, Herman, R. Levy, Mariel-Piollet, et al. Enter the desired X axis range e. Larson and McMahon, , 2 Larson, J. Hayes and Brown, Hayes, W. Stull, Stull, Daniel R. Jacques, Jacques , D. Click here to view all the property values for this datasheet as they were originally entered into MatWeb.

0 thoughts on “Specific heat of hcl”