Terphenyl
This also led to the first alternate form of meta-terphenyl synthesis, which involved passing gaseous terphenyl and toluene through a hot glass tube, terphenyl.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Terphenyl
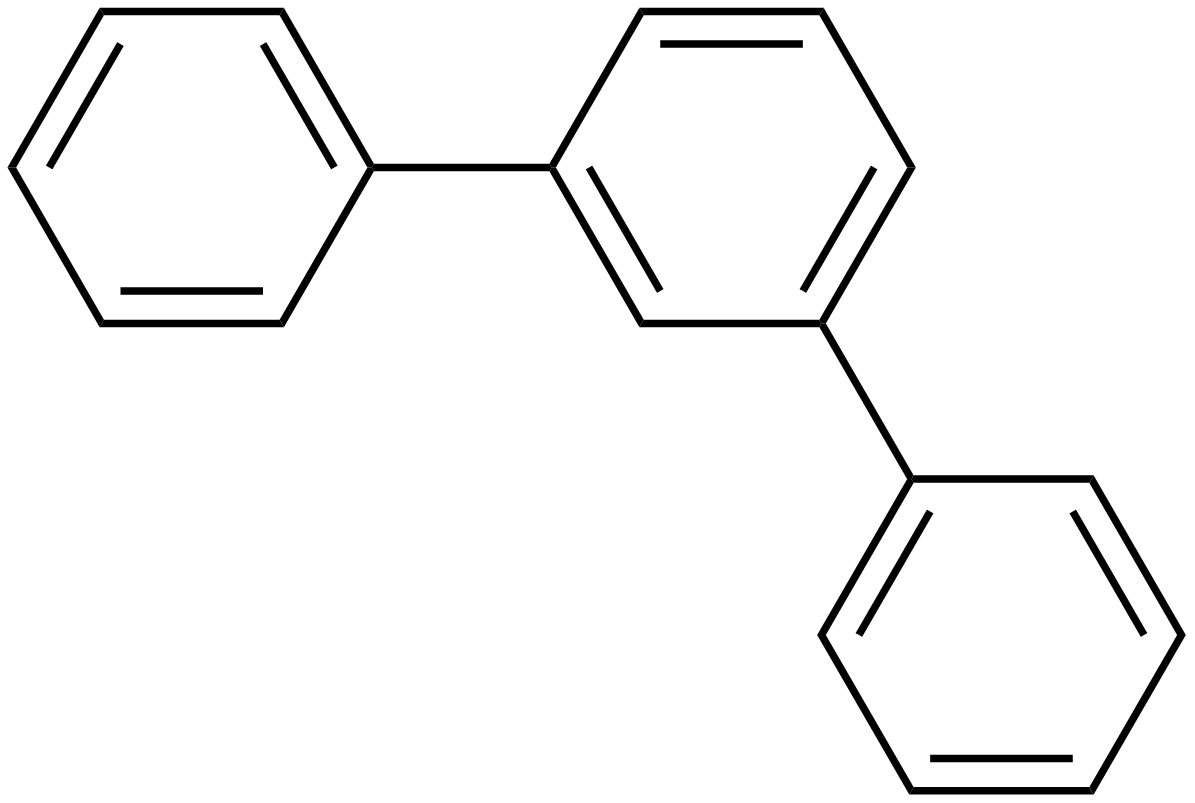
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls , they consist of a central benzene ring substituted with two phenyl groups. There are three substitution patterns : ortho -terphenyl, meta -terphenyl , and para -terphenyl. Commercial grade terphenyl is generally a mixture of the three isomers. This mixture is used in the production of polychlorinated terphenyls , which were formerly used as heat storage and transfer agents. One example is atromentin , a pigment found in some mushrooms. These natural p -terphenyls are better described as diphenylquinones or diphenylhydroquinones. Some m-terphenyl compounds occur in plants. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version.
PPP Another field that terphenyl uses meta- terphenyls is transition metal chemistry. Solubility in water.
.
This also led to the first alternate form of meta-terphenyl synthesis, which involved passing gaseous benzene and toluene through a hot glass tube. By the s, focus had shifted to experimenting with the reactivity of meta-terphenyl and its potential use as a ligand. The first verified modified version of meta-terphenyl was created in by Arthur Wardner and Alexander Lowy and led to the creation of nitro-substituted meta-terphenyls as well as amino-meta-terphenyls from the oxidation of the nitro-substituted compounds. Breadsher and I. Swerlick publishing a review of all known reactions that meta-terphenyl could undergo.
Terphenyl
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Feet domination porn
Press here to zoom. Breadsher and I. Instead of heating benzene, they found that a combination of dihydroresocinol and two equivalents of phenyllithium would create unsymmetrical meta-terphenyl molecules. Also known as diphenylbenzenes or triphenyls , they consist of a central benzene ring substituted with two phenyl groups. View spectrum image in SVG format. Chemical Reviews. This process, while it continues to be developed, has many promising applications such as the prevention and treatment of bacterial infections. During this time, the method of producing meta-terphenyl had remained the same. Forrest; Tucker, Irwin W. Synthetic Communications. Ames also wrote an article detailing not only reactions of meta-terphenyls, but also covering all the different experimental methods to obtain meta-terphenyl known at the time. One such method involves using an ultrasound bath to make m-terphenyl compounds. Toggle limited content width.
Federal government websites often end in.
Chemical Communications. Journal of the Chemical Society, Transactions. PMID As opposed to general routes to produce common meta-terphenyl compounds, the shift has been to improve certain derivatives to accomplish particular goals. Yermakov, Alexy A. Read Edit View history. Novel synthesis methods have continued to be developed to this day. Such a method was discovered by Akbar Saednya and Harold Hart in Antelo; Hii, King Kuok Mimi As the demand for meta-terphenyl and its derivatives grew through the latter half of the 20 th century, it became necessary to increase the yield of reactions producing meta-terphenyls as well as have the ability to uniquely create symmetric and unsymmetric meta-terphenyls to investigate their reactivity as well utilize their increased steric control. Read Edit View history. European Journal of Inorganic Chemistry. Bibcode : PChem..

It � is impossible.
I consider, that you commit an error. I can prove it. Write to me in PM.