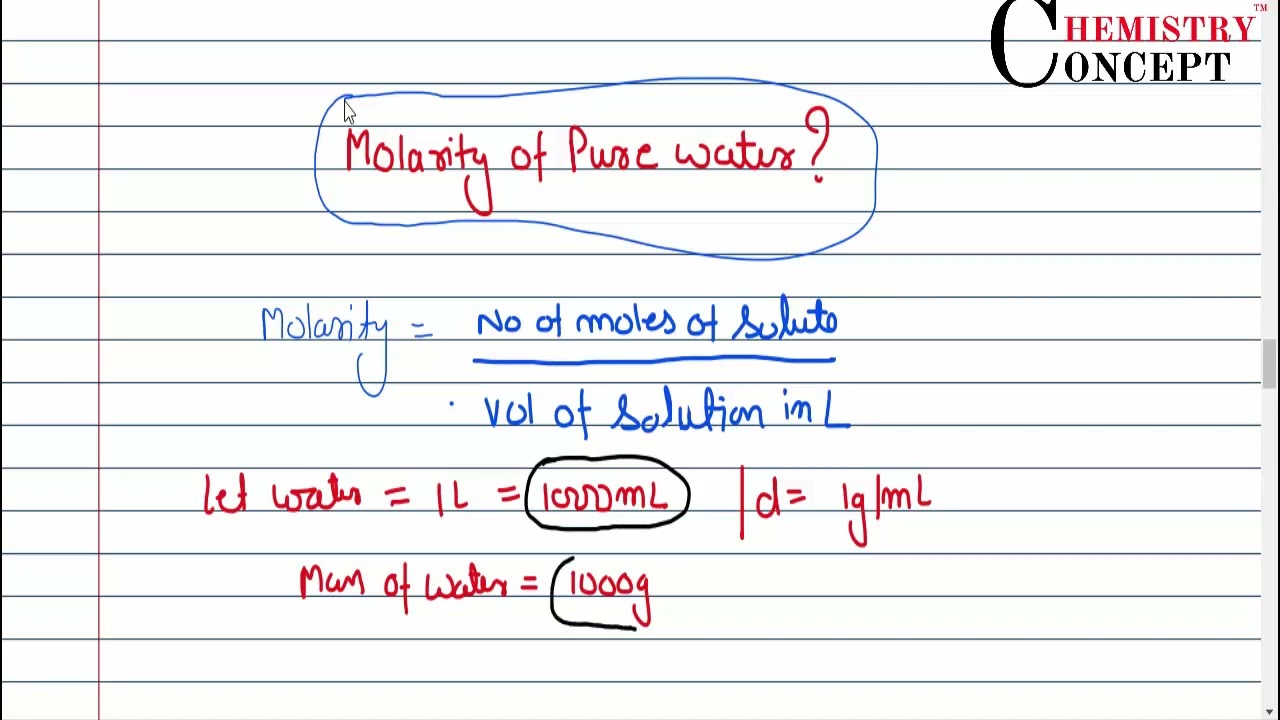
The molarity of pure water is
Explore this scientific idea in depth with a user-friendly guide that makes it easy for everyone to understand the chemistry of pure water. As a result, the molarity of pure water is
The topic would mainly focus on providing a brief narrative about the molarity of water H2O. The narrative would also focus on explaining in brief how water has molarity. The descriptive would also share a brief description of the Molarity of pure water. The chemical constituents, composition and important terms associated with the various biochemical and chemical compounds have been a crucial focus of study for a long time. These findings have then allowed us to discover different uses, and properties and uncover new facts which have further contributed to experimentation and helped in the scientific progression of various important resources. One such important resource is water, whose pure water percentage is mostly found in natural reservoirs like lakes, ponds, etc.
The molarity of pure water is
.
The descriptive would also share a brief description of the Molarity of pure water. PYQ Ethics case studies Booklet.
.
Another way of expressing concentration is to give the number of moles of solute per unit volume of solution. Of all the quantitative measures of concentration, molarity is the one used most frequently by chemists. Molarity is defined as the number of moles of solute per liter of solution. Chemists also use square brackets to indicate a reference to the molarity of a substance. Solution concentrations expressed in molarity are the easiest to perform calculations with, but the most difficult to make in the lab. Such concentration units are useful for discussing chemical reactions in which a solute is a product or a reactant. Molar mass can then be used as a conversion factor to convert amounts in moles to amounts in grams. For example, if you have 1.
The molarity of pure water is
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles. You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. To understand the topic as a whole, you will want to learn the mole definition, read a paragraph about the molarity units, as well as read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, we prepared for you some interesting examples of molar solutions and a short step-by-step tutorial on how to calculate the molarity of a concentrated solution. At the end, you can learn the titration definition and discover how to find the molar concentration using the titration process! The molarity calculator is straightforward and convenient, and you will find that out soon. But did you know how significant calculating molarity is?
Lord of the rings decor
Hence the molarity. The finding that water has molarity helps us understand the indivi Hence moles of a substance can be found by dividing the given mass of the solute by its calculated molecular mass. UPSC Toppers. Now we obtain the mass or weight of pure water to be g. A Jain responds to Meat Ban in Mumbai. The time encompassing experimentation on water has led to the discovery of important facts like the finding of the molarity of H 2 O. Load More. Thus now applying the formula to find moles gives,. Get started with your UPSC preparation today. All Business Economy1 Finance. The term solute describes the amount of solid substance present as a part of its solution. It is artificially obtained by evaporation of all the dissolved substances in a water sample. Your email address will not be published.
In preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances. Similar to a pure substance, the relative composition of a mixture plays an important role in determining its properties.
All Business Economy1 Finance. The topic would mainly focus on providing a brief narrative about the molarity of water H2O. Popular This Week. The term solute describes the amount of solid substance present as a part of its solution. Let us learn about the difference between bonding molecular orbital and antibonding molecular orbital with the help of bonding and antibonding orbital. Working of Surfactants Chemical compounds that are used to reduce the surface tension among various compounds are called surfactants. These findings have then allowed us to discover different uses, and properties and uncover new facts which have further contributed to experimentation and helped in the scientific progression of various important resources. Get started with your UPSC preparation today. Leave a Reply Cancel reply Your email address will not be published. The condition of water has molarity and its finding helps us know about the specific amount of any element or the quantity of compound in a solution. Share on Facebook Share on X.

I consider, that you are not right. I am assured. Write to me in PM.
I apologise, but it does not approach me. There are other variants?