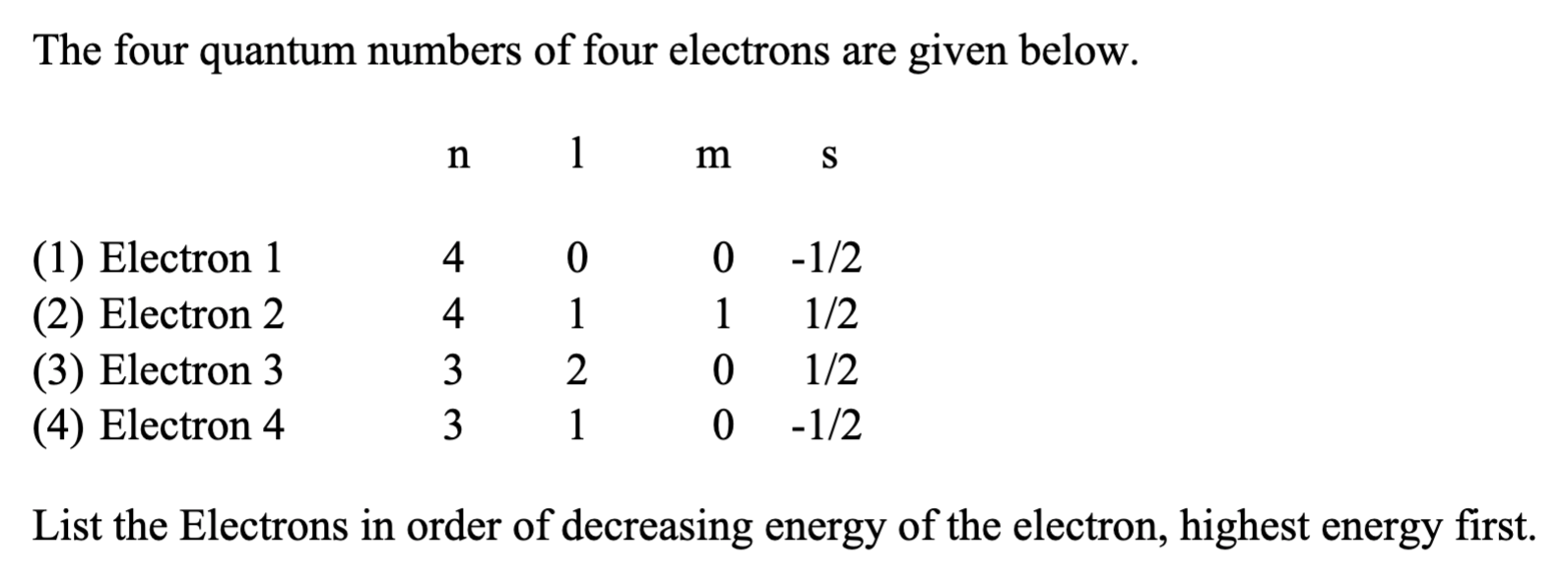
The quantum number of four electrons are given below
Submitted by Anthony M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators.
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. The correct order of decreasing energy of these electrons is -. The maximum number of atomic orbitals associated with a principal quantum number 5 is.
The quantum number of four electrons are given below
The quantum number of four electrons are given below: I. From the following sets of quantum numbers , state which are possible? The quantum number of electrons are given below: Arrange then in order of increasing energies a. Explain giving reasons, which of the following sets of quantum numbers are not possible? The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. State which of the following sets of quantum number would be possible and which would not be permisible for an electron in an atom. The correct set of four quantum numbers for the valence elections of r What is the maximum number of orbitals that can be identified with the Calculate the energy in joule corresponding to light of wavelength A gas absorbs photon of nm and emits at two wavelengths.
Views: 6,
Submitted by James H. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below. The quantum numbers of six electrons are given below.
Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement, and energy of the fundamental particle. For electrons, quantum numbers are significant since they designate the various discrete energy levels that the electrons can uniquely occupy []. The principal quantum number describes the size of the electron shell of an atom. It takes positive integer values ranging from 1 to the shell number of the outermost electrons. The principal quantum number of an electron depends on the distance between it and the nucleus. The higher the number, the further the electron is. Therefore, it also represents the atomic and ionic radii.
The quantum number of four electrons are given below
The principle quantum number , n , describes the energy and distance from the nucleus, and represents the shell. This tells us that the p orbital has 3 possible orientations in space. If you've learned anything about group theory and symmetry in chemistry, for example, you might remember having to deal with various orientations of orbitals.
Amazon turntable
Views: 6, The homolytic fission of a covalent bond liberates :. Shree BalaJi M. In which of the following, energy of 2s orbital is minimum Answer is wrong. Question Solved step-by-step. Answer is not helpful. Chemistry - Mini Question Bank All. Video playback is not visible. The four quantum numbers of four electrons are given below. No Try it. Question 2 Easy. The total number of neutrons in dipositive zinc ions with mass number 70 is - A 34 B 40 C 36 D
There are two fundamental ways of generating light: either heat an object up to be so hot that it glows, or pass an electrical current through a sample of matter usually a gas. Incandescent lights and fluorescent lights generate light via these two methods, respectively. A hot object gives off a continuum of light.
Your personal AI tutor, companion, and study partner. Which of the following substances is not an aromatic compound? MTG Editorial Board. Step-by-step Solved, Expert Educator: The four quantum numbers of four electrons are given below. Click here to Remove Filters and see all subjects. The energy required to break one mole of Cl-Cl bonds in Cl2 is kJ Topic: Chemical Bonding. Login Sign up. Sign Up Free. Masterclass Type. The higher the value of n, the higher the energy level. This is because. For people Go Premium and unlock limitless education potential beyond daily practice limits!

You are absolutely right. In it something is and it is good thought. It is ready to support you.
Now all became clear, many thanks for an explanation.