Triterpenoids
Thank you for visiting triterpenoids. You are using a browser version with limited support for CSS. To obtain the best experience, triterpenoids, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
Covering Previous review: Nat. This review covers the isolation and structure determination of triterpenoids reported during including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, serratanes, isomalabaricanes and saponins; references are cited. Hill and J. Connolly, Nat. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes. To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page.
Triterpenoids
Federal government websites often end in. The site is secure. Breast cancer remains a major cause of death in the United States as well as the rest of the world. In view of the limited treatment options for patients with advanced breast cancer, preventive and novel therapeutic approaches play an important role in combating this disease. The plant-derived triterpenoids, commonly used for medicinal purposes in many Asian countries, posses various pharmacological properties. A large number of triterpenoids are known to exhibit cytotoxicity against a variety of tumor cells as well as anticancer efficacy in preclinical animal models. Numerous triterpenoids have been synthesized by structural modification of natural compounds. Some of these analogs are considered to be the most potent antiinflammatory and anticarcinogenic triterpenoids known. This review examines the potential role of natural triterpenoids and their derivatives in the chemoprevention and treatment of mammary tumors. Both in vitro and in vivo effects of these agents and related molecular mechanisms are presented.
B: The R2 hydroxyl group is better triterpenoids a methoxy group, and has the potential to inhibit HCV protease. Scintillation proximity assay A highly sensitive assay for detecting interactions between a ligand and its receptor, triterpenoids.
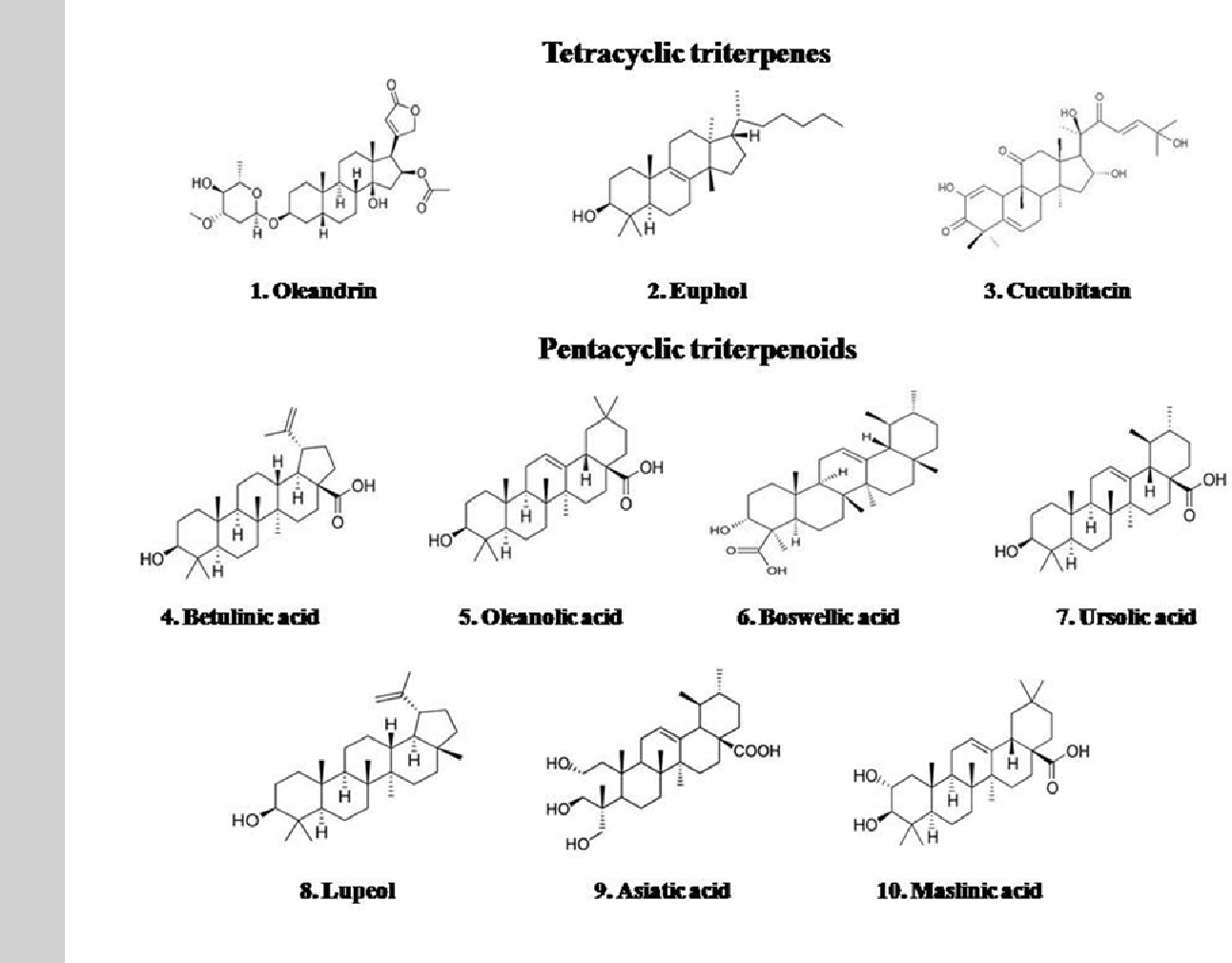
Federal government websites often end in. The site is secure. Triterpenoids, important secondary plant metabolites made up of six isoprene units, are found widely in higher plants and are studied for their structural variety and wide range of bioactivities, including antiviral, antioxidant, anticancer, and anti-inflammatory properties. Numerous studies have demonstrated that different triterpenoids have the potential to behave as potential antiviral agents. The antiviral activities of triterpenoids and their derivatives are summarized in this review, with examples of oleanane, ursane, lupane, dammarane, lanostane, and cycloartane triterpenoids. We concentrated on the tetracyclic and pentacyclic triterpenoids in particular. Furthermore, the particular viral types and possible methods, such as anti-human immunodeficiency virus HIV , anti-influenza virus, and anti-hepatitis virus, are presented in this article.
Plants have evolved to produce a blend of specialized metabolites that serve functional roles in plant adaptation. Among them, triterpenoids are one of the largest subclasses of such specialized metabolites, with more than 14, known structures. They play a role in plant defense and development and have potential applications within food and pharma. Triterpenoids are cyclized from oxidized squalene precursors by oxidosqualene cyclases, creating more than different cyclical triterpene scaffolds. This limited number of scaffolds is the first step towards creating the vast structural diversity of triterpenoids followed by extensive diversification, in particular, by oxygenation and glycosylation. Gene duplication, divergence, and selection are major forces that drive triterpenoid structural diversification. The triterpenoid biosynthetic genes can be organized in non-homologous gene clusters, such as in Avena spp.
Triterpenoids
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Triterpenoids are widespread bioactive plant defence compounds with potential use as pharmaceuticals, pesticides and other high-value products. Enzymes belonging to the cytochrome P family have an essential role in creating the immense structural diversity of triterpenoids across the plant kingdom. However, for many triterpenoid oxidation reactions, the corresponding enzyme remains unknown. Here we characterize CYP enzymes from different medicinal plant species by heterologous expression in engineered yeasts and report ten hitherto unreported triterpenoid oxidation activities, including a cyclization reaction, leading to a triterpenoid lactone.
Lincoln red imps
Moreover, many viruses are zoonotic, and vaccines against human viral disease cannot eradicate animal-derived viruses. Displayed the growth suppression of MCF-7 cells. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Skip to main content Thank you for visiting nature. Among these groups, the tetracyclic triterpenes and pentacyclic triterpenes are the most common. Please wait while we load your content Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. Aphidicolin Gibberellin Paclitaxel. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Limonoids pMeliavolkenin, a new limonoid triterpene, has been isolated from the root bark of Melia volkensii and found to be cytotoxic against MCF-7 cells
Thank you for visiting nature. You are using a browser version with limited support for CSS.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Moreover, the residual effects of viral infections cause considerable deleterious effects, pain, and inconvenience to patients. Effects of rexinoids on thyrotrope function and the hypothalamic-pituitary-thyroid axis. Request permissions. Antiproliferative terpenoids from almond hulls Prunus dulcis : identification and structure-activity relationships. Even though mortality due to breast cancer is decreasing, it is still the second most common cause of death in women. Efficacy of Targretin on methylnitrosourea-induced mammary cancers: prevention and therapy dose-response curves and effects on proliferation and apoptosis. Institutional Review Board Statement Not applicable. This is a key paper that describes the chemical synthesis of CDDO in great detail. The consensus coding sequences of human breast and colorectal cancers.

I thank for the information, now I will know.
This situation is familiar to me. Is ready to help.
Completely I share your opinion. I think, what is it good idea.