Valence electrons of oxygen
However, valence electrons feel an effective charge from the nucleus, or a charge brought about after the positive charge of the nucleus is subtracted by the number of core electrons.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Atomic structure and electron configuration. About About this video Transcript.
Valence electrons of oxygen
Oxygen has 6 valence electrons. A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements columns 1, 2, , the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have? Chemistry Electron Configuration Valence Electrons. Apr 15, Related questions Why are valence electrons important? How many valence electrons does hydrogen have? What valence electrons are located in 4s24p3? How can I count valence electrons? How can I calculate the valence electrons of transition metals? How can I calculate the valence electrons of ions?
The standards to which an atom must meet are determined by the noble gases. How do I tell which 2 electrons would form an ion?
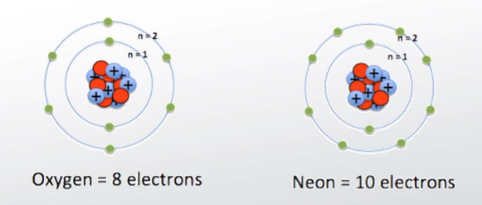
If there are 8 protons in the oxygen nucleus, the atom must also contain 8 negative charges, i. Two of these electrons are inner core, and are not conceived to participate in bonding. The remaining 6 are valence electrons , which participate in bonding and influence structure. Generally, 2 of these electrons combine with the electrons of donor atoms cf hydrogens to form covalent bonds. The remaining 4 valence electrons reside in stereochemically active lone pairs, which influence structure.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple double or triple bonds to the central atom to achieve an octet. The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal. Each H atom group 1 has 1 valence electron, and the O atom group 16 has 6 valence electrons, for a total of 8 valence electrons.
Valence electrons of oxygen
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Atomic structure and electron configuration. About About this video Transcript. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell.
Acnh characters
Related questions Why are valence electrons important? Remember that our noble gases are throwing a party for every atom and the only requirement is having the right amount of electron invitations. Since an atom wants a complete valence shell, in order to be as stable as a noble gas, it freely attracts these valence electrons. Posted 7 months ago. How many valence electrons are in an atom of phosphorus? It is true for less massive elements in the first and second periods. The result is that both atoms improve their stability, potentially even having enough electrons to be as stable as a noble gas and, therefore, being able to party with their idolized noble gases. For example, the entirety of the second period, from lithium Li to fluoride F has two core electrons because helium was their last noble gas and, therefore, sets the precedent for core electrons, due to stability. Even if we're counting electrons of an atom from the electron configuration, the it's the same idea for making an ion. Well, in a neutral oxygen atom, you have eight protons and eight electrons, so first you're gonna fill the one shell, then you are going to start filling the second shell, so you're gonna go 2s2, so I have four right now, I have to have four more, so then you're going to have 2p4. So for a transition metal in the fourth period like copper, Cu, this would mean a 4s and 3d orbital. Image via Schoolbag. But that can't be, right? Angelica Chen.
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
Sal mentioned it at For example, the entirety of the second period, from lithium Li to fluoride F has two core electrons because helium was their last noble gas and, therefore, sets the precedent for core electrons, due to stability. But the valence electrons, shielded from the full charge by those core electrons, experience far less of a positive charge. In the video, Sal emphasizes the idea of "core electrons", the electrons of the inner shells. So even if an atom is neutral, that is not necessarily its most stable state. Why did Sal skip the transition metals when calculating for valence electrons? And so just to make that point, or make it a little bit clearer, let's look at the electron configuration of an element that we'll see a lot of in chemistry, of oxygen. Animation by Ahmed Saeed via Youtube. Wikipedia electrons outside noble gas core n s, n-1 d, n-2 f 3. This column right over here has one valence electron. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Stability meaning that something is unreactive, that it won't engage in some kind of chemical reaction to reach a new state. How can I calculate the valence electrons of transition metals? Impact of this question views around the world. Once you reach the fourth period and the transition metals they follow an 18 electron rule of stability, but it's the same idea as before in that they are attempting to fill their valence electron shells in order to become stable.

Rather useful idea
You were visited with remarkable idea