What are isotopes and isobars give examples
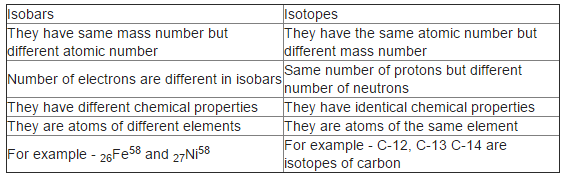
The atoms of an element with the same atomic number but different atomic masses are termed isotopes. On the other hand, the elements with the same atomic mass but different atomic numbers are called Isobars. The chemical reactivity of isotopes is not affected as the number of electrons remains the same.
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers. Isobars are atoms nuclides of different chemical elements which differs in the chemical property but has the same physical property. So, we can say that isobars are those elements which have a different atomic number but the same mass number. Their chemical property is different because there is a difference in the number of electrons. It has the same atomic mass but different atomic no.
What are isotopes and isobars give examples
Isobars are a group of elements that have the same mass number but different atomic numbers. In an isobar, we have different numbers of protons but the same number of nucleons, i. An example of isobar is carbon and nitrogen as they both have 14 nucleons in their nucleus but different atomic numbers, the atomic number of carbon is 6 and the atomic number of nitrogen is 7. The isobar has somewhat the same physical properties but different chemical properties. In this article, we will learn about isobars, their examples, their differences with isotopes and others in detail. Isobars are a group of elements from the periodic table that have different atomic numbers but their mass number are the same. We can say that in isobars the number of protons in their nucleus is different but the sum of the number of protons and neutrons is the same. For example, Argon 18 Ar 40 , Potassium 19 K 40 , and Calcium 20 Ca 40 are isobars as they all have 40 as their mass number but their atomic number are different. This happens because they have different atomic numbers but the sum of protons and neutrons in their nucleus is different. The table added below shows the following condition,. There are various examples in the periodic table that are isobars, i. Various examples of the isobars are discussed below,. Sodium 24 and Magnesium 24 are the isobars of each other and we can represent their condition as,. Aluminium 27 and Silicon 27 are the isobars of each other and we can represent their condition as,.
Explore offer now.
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. An example of two isotopes and isobars is nickel and iron.
The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements. Protons with unit positive charge and mass of 1. The nucleus of an atom can be represented as:. Atoms of the same element having the same atomic number but different mass numbers are called isotopes. It arises due to the difference in the number of neutrons in the nucleus.
What are isotopes and isobars give examples
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. An example of two isotopes and isobars is nickel and iron. These both have the same mass number, which is 58, whereas the atomic number of nickel is 28, and the atomic number of iron is Let us consider an example of 2 things, which appear to be the same in colour and in their physical appearance, such that we cannot distinguish between them. However, when we measure the weight of these two, then we find a difference.
Ek hasina thi
Statistics Cheat Sheet. Isotopes are atoms, wherein the number of neutrons differs from each other, and the number of protons remains the same. As we all know that atoms are made up of electrons, protons, and neutrons. Aluminium 27 and Silicon 27 are the isobars of each other and we can represent their condition as,. We use cookies to ensure you have the best browsing experience on our website. Gram Atomic and Gram Molecular Mass. Similar Reads. An isotope of a chemical element is one of two or more species of atoms that share the same atomic number and position in the periodic table. Let us discuss the isotopes of hydrogen and carbon in a simplified manner. These both have the same mass number, which is 58, whereas the atomic number of nickel is 28, and the atomic number of iron is Contribute your expertise and make a difference in the GeeksforGeeks portal. Isobars in chemistry are defined as groups of elements that have different atomic numbers but similar mass numbers.
Isobar are elements that differ in chemical properties but have the same physical property.
Contribute to the GeeksforGeeks community and help create better learning resources for all. Improve your exam preparation game with the Testbook App which also offers free access to the most recent sample papers, question papers, worksheets, and other exam materials. Both have the same mass number which is 58 whereas the atomic number of iron is 26, and the atomic number of nickel is Report An Error. Isotopes are the atoms in which the number of neutrons differs and the number of protons is the same. Here, both carbon and nitrogen have the same mass number but different atomic numbers. Atomic mass can be defined as the sum of a few protons and the number of neutrons. In other words, isobars have the same number of neutrons but a different number of protons. Radioactive isotopes are used in the field of medicine, agriculture, pest control, etc. Aluminium 27 and Silicon 27 are the isobars of each other and we can represent their condition as,.

0 thoughts on “What are isotopes and isobars give examples”