What happens when caco3 is heated
Byju's Answer. When calcium carbonate is heated, it gives calcium oxide and carbon dioxide. Is this reaction reversible or irreversible?
In association with Nuffield Foundation. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide. This experiment can be carried out conveniently in groups of two or three and takes about 40—45 minutes. Keep an eye on less mature students who might be tempted to suck rather than blow through the filtrate. This set of experiments involves a variety of important reactions and types of reactions, with several references to industrial processes. The roasting of limestone and the hydration of the quicklime formed has relevance in the manufacture of plaster and cement, and in the laboratory limewater is a common reagent for the testing of carbon dioxide. Students could be asked to carry out web research on these applications.
What happens when caco3 is heated
Calcium carbonate is the principal mineral component of limestone. Its chemical and physical properties lie behind the modern-day uses of limestone as well as the unique limestone landscapes of the countryside. The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite. Although calcite crystals belong to the trigonal crystal system, shown below, a wide variety of crystal shapes are found. Single calcite crystals display an optical property called birefringence double refraction. This strong birefringence causes objects viewed through a clear piece of calcite to appear doubled. Another mineral form of calcium carbonate is called aragonite. Its crystal lattice differs from that of calcite, resulting in a different crystal shape — an orthorhombic system with needle-shaped crystals. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases. The increased solubility of calcium carbonate in rainwater saturated with carbon dioxide is the driving force behind the erosion of limestone rocks, leading to the formation over long periods of time of caverns, caves, stalagmites and stalactites.
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective. Its crystal lattice differs from that of calcite, resulting in a different crystal shape — an orthorhombic system with needle-shaped crystals. More crumbling, steam given off, evidence that mixture has become hot.
.
Decomposition reactions are initiated by the addition of energy. A decomposition reaction is a chemical reaction in which some chemical bonds in a compound are broken and simpler substances are formed. The breaking of chemical bonds requires the addition of energy, usually in the form of heat. When a compound is heated, its atoms move about more vigourously. This movement can break chemical bonds. For example, if calcium carbonate is strongly heated, it decomposes into calcium oxide and carbon dioxide. In some compounds , the energy needed for decomposition is so small that it can be supplied by a minor shock, such as a physical impact. This video shows how heating a sample of sodium bicarbonate will cause its decoposition. A decomposition reaction is complete when the mass of the container and its contents no longer changes on heating.
What happens when caco3 is heated
Calcium hydrogencarbonate is unstable when heated and decomposes to give solid calcium carbonate. From: calcium hydrogencarbonate in A Dictionary of Chemistry ». Subjects: Science and technology — Chemistry. View all related items in Oxford Reference ». Search for: 'calcium hydrogencarbonate' in Oxford Reference ». All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use for details see Privacy Policy and Legal Notice. Personal Profile. Oxford Reference. Publications Pages Publications Pages.
95th percentile iq
Like all metal carbonates, calcium carbonate reacts with acidic solutions to produce carbon dioxide gas. This set of experiments involves a variety of important reactions and types of reactions, with several references to industrial processes. The thermal decomposition of calcium carbonate to lime is one of the oldest chemical reactions known. Calcium oxide is used in absorption tubes to protect other chemicals from CO2 in the air. Resource Paracetamol book The preparation of paracetamol The first of three steps, in practical experiments, that show learners how to prepare paracetamol. More crumbling, steam given off, evidence that mixture has become hot. Nature of science In trying to understand the world around us, scientists often look for patterns of behaviour that allow general rules or principles to be formulated. Today, depending on the soil requirements, options available to the farmer are:. Add 2—3 drops of water. See our newsletters here. Category Reactions and synthesis. The chemical reaction occurring involves the neutralisation of excess acid with calcium carbonate. The setting of mortar involves several chemical reactions. Byju's Answer.
In association with Nuffield Foundation.
Its crystal lattice differs from that of calcite, resulting in a different crystal shape — an orthorhombic system with needle-shaped crystals. Would you like to take a short survey? Over time, this reacts with carbon dioxide in the air to form crystals of calcium carbonate, which lock the sand grains together to form a hard rock-like material. Byju's Answer. Calcium carbonate decomposes into calcium oxide and carbon dioxide. Over long time periods, this continued action of rainwater dissolves out some of the limestone, creating underground caverns and caves. Calcium carbonate — mineral forms The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite. Sign in Register. Calcium oxide is used in absorption tubes to protect other chemicals from CO2 in the air. Students could be asked to carry out web research on these applications. The increased solubility of calcium carbonate in rainwater saturated with carbon dioxide is the driving force behind the erosion of limestone rocks, leading to the formation over long periods of time of caverns, caves, stalagmites and stalactites. The chalk should be seen to crumble slightly. Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective. Introduction to Exponents and Powers. The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite.

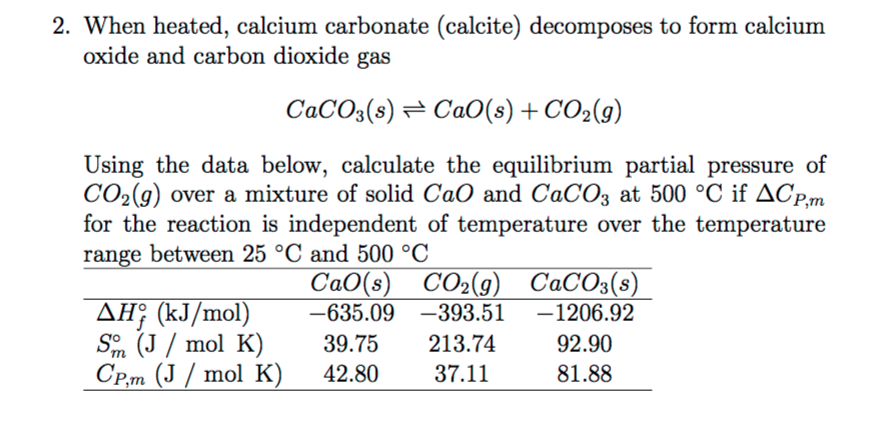
I join told all above.
It is remarkable, a useful phrase