What is the conjugate base of h2so4
What is the conjugate of H 2 SO 4?
Wiki User. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Conjugate base. HSO4 -. Nope, itsHSO H2SO4 is already a strong acid. If you mean what is the conjugate base, then the answer is HSO
What is the conjugate base of h2so4
Identify the acid, base, conjugate acid and conjugate base in the following reaction. HSO4" aq …. A: Acid Base chemistry. Q: Which statement is true of this chemical equation? Q: acid, base, conjugate acid, and conjugate base. Q: Identify the acid, base, conjugate acid, and conjugate base in the following reactions. A: According to Bronsted-Lowry concept an acid is a proton donor and a base is a proton acceptor. Q: What is the conjugate base of phosphoric acid? A: The acid given is phosphoric acid i. Q: Which of the following acids would have the strongest conjugate base? Acid Ka HIO3 1. A: Given : To find the strongest conjugate base from the given acids.
Open in App.
.
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test. Identify the correct sequence of the reactions. Byju's Answer. Open in App. Conjugate acid and base concept: According to Bronsted Lowry's theory, it is formed by the addition of conjugate acid and conjugate base in a chemical reaction. It is divided into two sections: When an acid is capable of donating a proton and there is an addition of a proton to the acid is known as conjugate acid. When a base is capable of accepting a proton and there is the removal of a proton from the acid is known as conjugate base.
What is the conjugate base of h2so4
See this Socratic answer. A conjugate base contains one less H atom and one more - charge than the acid that formed it. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions.
In the meantime en español
Ka for… A: The more ka value of acid the more acidic and the conjugatebase of that strong acid is weakest…. HC2H3O2 acetic acid c. They are the products of an acid-base reaction by the Bronsted-Lowry definition. A: Conjugate base is formed when acid losses proton. A: Ans given as follows. Q: Identify acid, base, conjugate acid, and conjugate base in the following reactio a. Publisher: John C. Q: Which statement is true of this chemical equation? John W. HF is an acid and F- is its conjugate base. A: Interpretation- conjugate base. A non-metallic element is converted into a compound X after a series of reactions.
On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction.
Conjugate acid and base concept: According to Bronsted Lowry's theory, it is formed by the addition of conjugate acid and conjugate base in a chemical reaction. Introductory Chemistry: A Foundation. Q: Identify the acid, base, conjugate acid, and conjugate base in the following reactions. See similar textbooks. Goode, David W. Knowledge Booster. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Chemistry: Principles and Practice. General, Organic, and Biological Chemistry. Bronsted Lowry Base In Inorganic Chemistry Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with. Still have questions? A: Conjugate base is formed when acid losses proton.

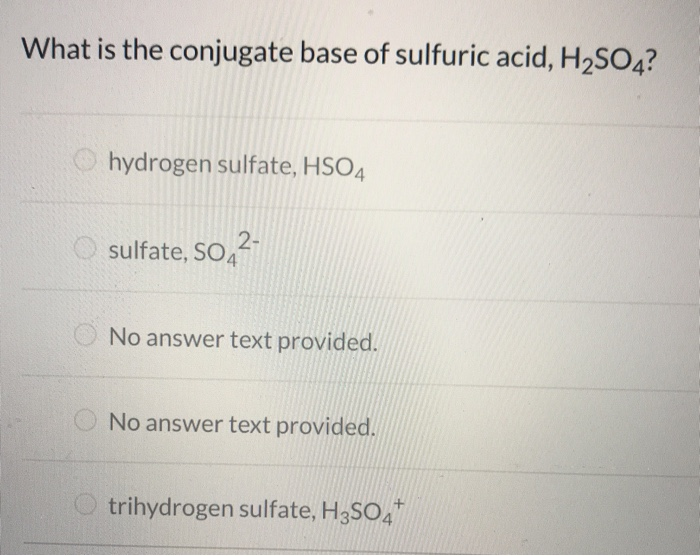
It is exact
Warm to you thanks for your help.
I am sorry, that has interfered... At me a similar situation. It is possible to discuss.