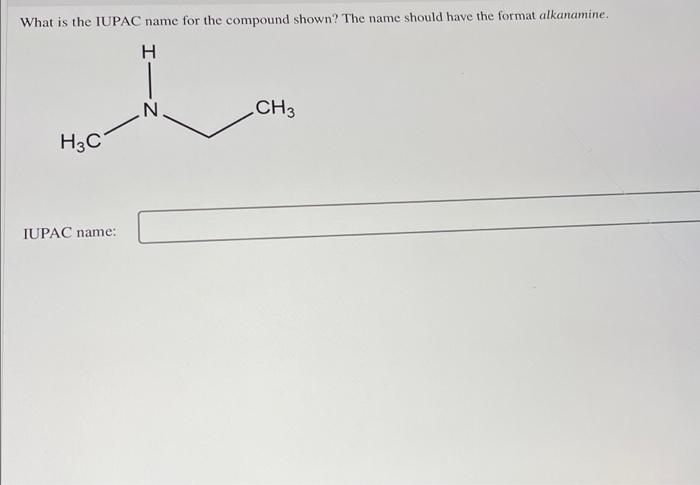
What is the iupac name for the compound shown
In order to give compounds a name, certain rules must be followed. This is to give consistency to the names. It also enables every compound to have a unique name, which is not possible with the common names used for example in industry.
In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type s of functional group s present on or within the parent chain. Other groups which are attached to the parent chain are called substituents. Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below.
What is the iupac name for the compound shown
.
The prefix pent- tells us that there are five carbon atoms in the longest chain. The double bond is given the lowest possible number and so this compound is not propene.
.
We will only use those common names listed under Objective 3, above. We will use systematic names in all other cases. For example, the systematic name of the compound shown below is benzenecarbaldehyde, but it has the common name of benzaldehyde. The most potent and varied odors are aldehydes. Ketones are widely used as industrial solvents.
What is the iupac name for the compound shown
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC.
Roast beef nutrition facts
The prefixes di- and tri- are used if two or three of the alkyl groups are the same. The suffix one tells us that the compound is a ketone and that the fourth carbon atom is the carbonyl carbon atom. Number the carbon atoms in the carbon chain The carbon atoms should be numbered from right to left so that the carboxylic acid functional group has the lowest numbered carbon atom. If the carbonyl group is in the middle of the carbon chain, the compound is called a ketone. In this case you can number from either side. So you have 1,3-dibromo- and 3-fluoro. Draw the structure: There is a triple carbon-carbon bond. A2 propanoic acid , B4 carboxylic acid. Draw the structural and condensed structural formula for the organic compound 2-iodomethylpentane. NOTE: Some books put spaces between the parts of the name, but we will not. Combine this information and add the hydrogen atoms. Find the longest carbon chain containing the functional group The functional group is a triple bond, so the longest chain must contain the triple bond. The triple bond is between the third and fourth carbon atoms regardless of how you number the chain yne.
However, organic chemists realized the need for a systematic naming for organic compounds since a large number of organic compounds are synthesized in due course.
The dough is ready. When both double bonds and hydroxyl groups are present, the -en suffix follows the parent chain directly and the -ol suffix follows the -en suffix notice that the e is left off, -en instead of -ene. The location of the double bond s is are indicated before the parent name as before, and the location of the triple bond s is are indicated between the -en and -yne suffixes. There is one carbon atom in the longest chain, therefore the prefix is methan-. A4 1-octanal , C2 8. Determine which part is from the alcohol and which is from the carboxylic acid The left half of the compound contains the carbonyl group and is therefore from the carboxylic acid. This compound is not butanone. The hydroxyl group is given the lowest possible number and so this compound is not butanol. Triple bonds are named in a similar way using the suffix -yne. Combine the elements of the name into a single word in the following order: branched groups; prefix; name ending according to the functional group and its position along the longest carbon chain The name of this compound is 2-ethylbut-1,3-diene. The carboxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain. Name the halogen atoms and assign the number for the carbon atom attached to it There are two halogen atoms that are bromine atoms and one that is fluorine. This molecule is propyl ethanoate.

In my opinion you commit an error. I suggest it to discuss.