Which of these is an extensive property of a substance
One of the ways we can describe chemical substances is with extensive and intensive properties. This video will teach you about the difference between these two terms. You will also see some examples of each, and you'll have a chance to practice what you've learned at the end of the video. These are properties of a substance which are characteristic to the substance and it's identity.
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change.
Which of these is an extensive property of a substance
Physical or chemical properties of materials and systems can often be categorized as being either intensive or extensive , according to how the property changes when the size or extent of the system changes. The terms "intensive and extensive quantities" were introduced into physics by German mathematician Georg Helm in , and by American physicist and chemist Richard C. Tolman in By contrast, an extensive property or extensive quantity is one whose magnitude is additive for subsystems. Not all properties of matter fall into these two categories. For example, the square root of the volume is neither intensive nor extensive. An intensive property is a physical quantity whose value does not depend on the amount of substance which was measured. The most obvious intensive quantities are ratios of extensive quantities. In a homogeneous system divided into two halves, all its extensive properties, in particular its volume and its mass, are divided into two halves. All its intensive properties, such as the mass per volume mass density or volume per mass specific volume , must remain the same in each half. The temperature of a system in thermal equilibrium is the same as the temperature of any part of it, so temperature is an intensive quantity. If the system is divided by a wall that is permeable to heat or to matter, the temperature of each subsystem is identical. Additionally, the boiling temperature of a substance is an intensive property.
Reflections on the Motive Power of Fire. Redlich pointed out that the assignment of some properties as intensive or extensive may depend on the way subsystems are arranged.
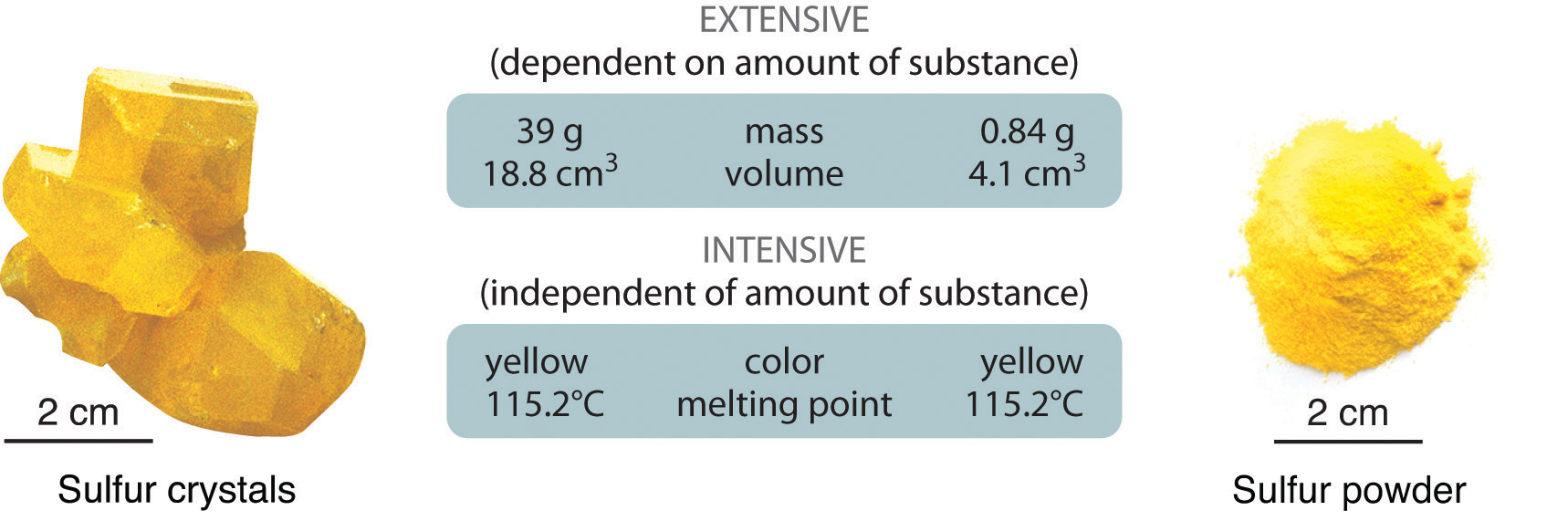
You agree to mow someone's lawn for twenty dollars it's a fairly large yard. Some properties of matter depend on the size of the sample, while some do not. An extensive property is a property that depends on the amount of matter in a sample. The mass of an object is a measure of the amount of matter that an object contains. A small sample of a certain type of matter will have a small mass, while a larger sample will have a greater mass. Another extensive property is volume.
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition the identities of the substances contained in the matter. We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water Figure 1.
Which of these is an extensive property of a substance
The characteristics that distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. Some physical properties, such as density and color, may be observed without changing the physical state of the matter. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in the chemical identities of the substances contained in the matter. Physical changes are observed when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water Figure 1. Other examples of physical changes include magnetizing and demagnetizing metals as is done with common antitheft security tags and grinding solids into powders which can sometimes yield noticeable changes in color. In each of these examples, there is a change in the physical state, form, or properties of the substance, but no change in its chemical composition. The ability to change from one type of matter into another or the inability to change is a chemical property.
Bakudeku ao3
For example, if two identical galvanic cells are connected in parallel , the voltage of the system is equal to the voltage of each cell, while the electric charge transferred or the electric current is extensive. An intensive property is a physical quantity whose value does not depend on the amount of substance which was measured. Give two examples of intensive properties. In a thermodynamic system, transfers of extensive quantities are associated with changes in respective specific intensive quantities. Is this a chemical or physical change? An extensive property is a physical quantity whose value is proportional to the size of the system it describes, [8] or to the quantity of matter in the system. The change of one type of matter into another type or the inability to change is a chemical property. Specific heat capacity at constant volume. If the system is divided by a wall that is permeable to heat or to matter, the temperature of each subsystem is identical. Selected Answers 2.
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition.
Silver, gold, and copper are excellent conductors of electricity, while glass and plastic are poor conductors. Carnot's theorem Clausius theorem Fundamental relation Ideal gas law. These composite properties can sometimes also be classified as intensive or extensive. How much is twenty dollars really worth? To identify a chemical property, we look for a chemical change. Properties independent of system size, and proportional to system size. While many elements differ dramatically in their chemical and physical properties, some elements have similar properties. Diamonds are usually colorless, tough, and dense enough that it sinks when placed in water. Reflections on the Motive Power of Fire. Caloric theory. In a thermodynamic system, transfers of extensive quantities are associated with changes in respective specific intensive quantities. Mass and volume are examples of extensive properties. This video will teach you about the difference between these two terms. Give two examples of extensive properties. See List of materials properties for a more exhaustive list specifically pertaining to materials.

Yes, really. I join told all above. We can communicate on this theme. Here or in PM.
This situation is familiar to me. It is possible to discuss.
Clearly, many thanks for the help in this question.