Xef2 lewis dot structure
This article explains the XeF2 Lewis structure and its characteristics. XeF2 itself is a powerful substance that can both fluorinate and oxidize.
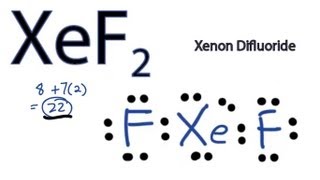
Transcript: Hi, this is Dr. Let's do the XeF2 Lewis structure. Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2. That'll give us a total of 8 plus 22 valence electrons. Xenon is the least electronegative.
Xef2 lewis dot structure
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 2 Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to XeF 2 for more practice. There are a total of 22 valence electrons in the Lewis structure for XeF2. BrF 5. BrO 3 -. ClO -.
Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2. Identify the central atom. ClO .
.
Ready to learn how to draw the lewis structure of XeF2? Here, I have explained 5 simple steps to draw the lewis dot structure of XeF2 along with images. The Xenon atom Xe is at the center and it is surrounded by 2 Fluorine atoms F. The Xenon atom has 3 lone pairs and both the Fluorine atoms also have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of XeF2. Here, the given molecule is XeF2 xenon difluoride.
Xef2 lewis dot structure
XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride. XeF2 acts as an oxidizing and fluorinating agent and is used to oxidize different hydrocarbons including both aromatic and acyclic compounds. Not only this, but this fluoride compound can also be used to etch silicon to form silicon tetrafluoride SiF4 without any external energy application. If you are thinking about what XeF2 looks like, it appears as a colorless-to-white crystalline solid with a density of around 4. This halide can cause some serious hazards like skin burns and major eye damage. Not only this, if inhaled or swallowed, it turns out to be fatal.
Bacsi online
PO 4 Check if the central atom has an octet. Determine the total number of valence electrons. The Lewis structure of XeF2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. Xenon, unlike other noble gases, can react and create different compounds like Xenon tetrafluoride XeF4 and Xenon hexafluoride XeF6. We draw Lewis Structures to predict: -the shape of a molecule. It shows xenon Xe as the central atom bonded to two fluorine F atoms. Opens New Window. XeF2 is a stable compound under the appropriate conditions and is commonly used in laboratory and industrial settings. XeF2 Lewis structure. What we'll do now is, we'll look at the formal charges to see if this is actually the structure that makes sense, or we maybe need that double bond. ClO 2 -. It is helpful if you: Try to draw the XeF 2 Lewis structure before watching the video. So on Fluorine right here, we have 7 valence electrons because it's in group 7 or 17 on the periodic table.
XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Out of these compounds, XeF2 is the most stable one.
So we still have two valence electrons left over, we still have a pair left. It is helpful if you: Try to draw the XeF 2 Lewis structure before watching the video. Calculate the formal charges for each atom in the molecule. The Lewis structure of XeF2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. ClO 4 -. Xenon is the least electronegative. The final Lewis structure of XeF2 should look like this:. For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. ClO 4 -. We draw Lewis Structures to predict: -the shape of a molecule.

I consider, that you are mistaken. I suggest it to discuss.
What good interlocutors :)
I join. I agree with told all above. We can communicate on this theme.