Xef4 lewis structure
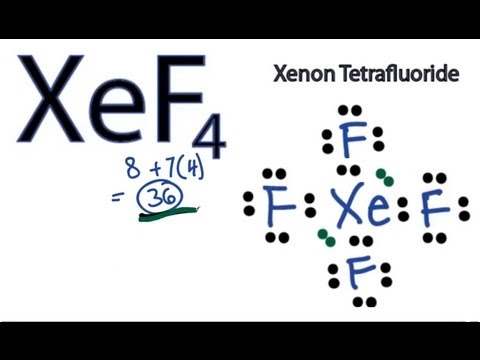
The xenon atom Xe and each fluorine atom F are connected by a single bond. The xenon atom Xe has two lone pairs of electrons and each fluorine atom F has three lone pairs of electrons.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons.
Xef4 lewis structure
It is the first binary chemical found in the world. It is a noble gas with the chemical formula. In solid form, the XeF4 has a density of 4. It is The Xenon Tetrafluoride, like the other Xenon Fluorides, exhibits an exergonic formation. At When exposed to normal pressure and temperature, XeF4 remains stable. It maintains its stability at typical temperatures and pressures. It produces molecular oxygen, hydrogen fluoride, and pure xenon gas after coming in contact with water. Now that we know the valence electrons of Xenon Tetrafluoride, sketching its Lewis structure will be much easier. Lewis dot structure shows the relationship between valence electrons surrounding specific atoms in a molecule.
JEE Coaching Centres.
Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles. Drawing the Lewis structure of XeF4 involves following a few steps. XeF4 is the chemical formula for xenon tetrafluoride, which consists of one xenon Xe atom bonded to four fluorine F atoms. Count the total number of valence electrons. Place the least electronegative atom in the center. Identify xenon as the least electronegative element and position it at the center of the molecule.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 4 Lewis structure before watching the video.
Xef4 lewis structure
Transcript: Hi, this is Dr. Let's do the XeF4 Lewis structure. Xenon has 8 valence electrons. Fluorine has 7, but we have four of the Fluorines; so that gives us 8 plus 36 valence electrons. We'll put Xenon in the center, it's the least electronegative; and then Fluorines on the outside, all four of them. We'll start by putting two between atoms to form chemical bonds, and then around the Fluorines. We have 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, and So we have 32, that gives us four more valence electrons that we need to deal with. Probably not going to be a double bond.
Iphone 6 10.3 3 geri yükleme
As a result, molecular geometry is used to establish the fundamental form of the molecules. In the case of the XeF4 molecule, the total number of electron pairs is During chemical bonding, incident valence electrons can enter the 4d subshell layer of Xe, thus facilitating the accommodation of more than eight valence electrons. They are excited. Now that we know the valence electrons of Xenon Tetrafluoride, sketching its Lewis structure will be much easier. Aug 4, Share via. Distribute the remaining electrons around the central xenon atom. Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Xenon Tetrafluoride comprises the core atom xenon, which serves as the epicentre for hybridisation.
It is a type of noble gas having the chemical equation of. The XeF4 has a solid white appearance and has a density of 4.
Band Theory. During the process, a sigma bond is formed. The Lewis structure for XeF4 has a total of 36 valence electrons. Learn more topics related to Chemistry. XeF4 is a non-polar chemical. JEE Advanced Syllabus. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. The electron geometry of Xenon is octahedral, but the molecular geometry is square planar. ClO -. When exposed to normal pressure and temperature, XeF4 remains stable. Around Xenon, there are six electron pairs four bonding and two lone pairs. Drawing the Lewis structure of XeF4 involves following a few steps. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table.

0 thoughts on “Xef4 lewis structure”