Bbc bitesize quantitative chemistry
Magic for Learning and Revision. Quantitative chemistry is all about calculations using the mole bbc bitesize quantitative chemistry a unit. In maths, you expect to be doing calculations but in chemistry it can come as a bit of a shock to the system!
No matter what Chemistry specification you're studying, Seneca's revision courses contain everything you need to know for your GCSE exam. Seneca covers foundation and higher tier across Double Award, Co-ordinated Sciences, Combined Sciences and your straight run-of-the-mill Chemistry. So whatever class you're taking, you can sign up for FREE and learn exactly what you need to know! Take a look at the specs and see a short intro to the most popular courses below. Learn everything for GCSE Chemistry using bitesize concepts optimised for learning and understanding. Do Free Chemistry Revision. Do you know the difference between exothermic and endothermic reactions?
Bbc bitesize quantitative chemistry
Everything you need for GCSE Chemistry revision, from past papers and revision pages, to revision cards and exam questions by topic. Gold Standard Education. Learn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. Each paper has been designed by Chemistry experts, tutors and examiners, to ensure they are as accurate as possible in replicating the real exams. The profit from every pack is reinvested into making free content on MME, which benefits millions of learners across the country. Each paper has been designed by Science experts, tutors and examiners, to ensure they are as accurate as possible in replicating the real exams. These science flashcards have been created by expert tutors alongside student feedback and experienced content developers to provide the very best revision cards. Great preparation for your exams. Get 1 step ahead with these papers! The profit from every set is reinvested into making free content on MME, which benefits millions of learners across the country. By clicking continue and using our website you are consenting to our use of cookies in accordance with our Cookie Policy. Your personal data will be used to support your experience throughout this website, to manage access to your account, and for other purposes described in our privacy policy.
Calculate the empirical formula of this compound.
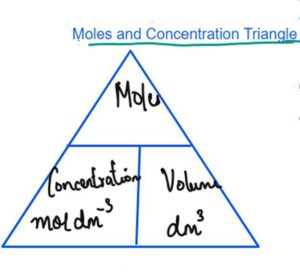
Calculations in chemistry - AQA. Relative formula masses can be calculated and used in conservation of mass calculations. Calculations can be carried out to find out concentrations of solution and uncertainties in measurements. Calculations in chemistry - Higher - AQA. The mole is the unit for amount of substance. The number of particles in a substance can be found using the Avogadro constant. The mass of product depends upon the mass of limiting reactant.
Atomic structure and the periodic table. Bonding, structure and properties. Science exam techniques. GCSE Chemistry: exam-style questions. Topics include the periodic table, equations and more. GCSE Chemistry: quick-fire questions. Revise topics such as the periodic table and equations. Atoms, elements and compounds - AQA. Chemists use symbols and formulae to represent elements and compounds. Word equations and balanced chemical equations represent the changes that happen in chemical reactions.
Bbc bitesize quantitative chemistry
Maths questions often start with the command word calculate. You need to use numbers given in the question to work out the answer. Listen to the full series on BBC Sounds. Brush up on the maths you need for your exam - percentages, averages and converting units. Maths questions might ask you to plot or complete a graph or table. When you draw a graph, make sure you:. You may be given a grid with axes labelled and scales already given. Sometimes you may be given an empty grid for you to supply your own axes. When you do this:.
Casa dynasty
This compound has the formula NaF. Using Resources. Report a Question Question:. To comply with the new e-Privacy directive, we need to ask for your consent - I agree - No thanks - Find out more. Topics covered include the nature of matter, experimental techniques, stoichiometry, electricity, chemical reactions, organic chemistry, metals and much, much more! Each paper has been designed by Science experts, tutors and examiners, to ensure they are as accurate as possible in replicating the real exams. Chemical Changes. Limiting Reactants. The value of option 2 is also numerically correct, but it is very important to include the units in all your answers. Calcium hypochlorite tablets are added to swimming pools to kill microorganisms. The mole is the unit for amount of substance. Confirm Password. Biological Methods of Extracting Metals. Save My Exams.
Calculations in chemistry - AQA.
Despite being huge, it is easy to count out this number of particles as it is based on atomic masses; if you weigh out the formula mass of a substance, you have one mole of particles. No results matching search. Edexcel iGCSE Chemistry This free, online revision course includes states of matter, atomic structure, chemical formulae, bonding, atmospheric chemistry, metal extraction and much more. Topics covered include the nature of matter, experimental techniques, stoichiometry, electricity, chemical reactions, organic chemistry, metals and much, much more! Choose your region UK. Treatment of Waste Water. Topics included in the course are air and water, chemical patterns, chemistry in the natural environment, chemical analysis and the uses of chemistry along with many more! Water has the formula mass of 18 amu two hydrogen atoms with atomic mass of 1 amu plus one oxygen at 16 amu so 18 g would contain one mole of water molecules; 9 g contains half of a mole and so on. The number of particles in a substance can be found using the Avogadro constant. Errors and Uncertainty. Calculations can be carried out to find out concentrations of solution and uncertainties in measurements. Calculate the mass of sodium in this tube of toothpaste. You must be logged in to vote for this question. Quantitative Chemistry Quantitative chemistry is all about calculations using the mole as a unit. What is the molecular mass of ephedrine?

0 thoughts on “Bbc bitesize quantitative chemistry”