Bohr rutherford diagram phosphorus
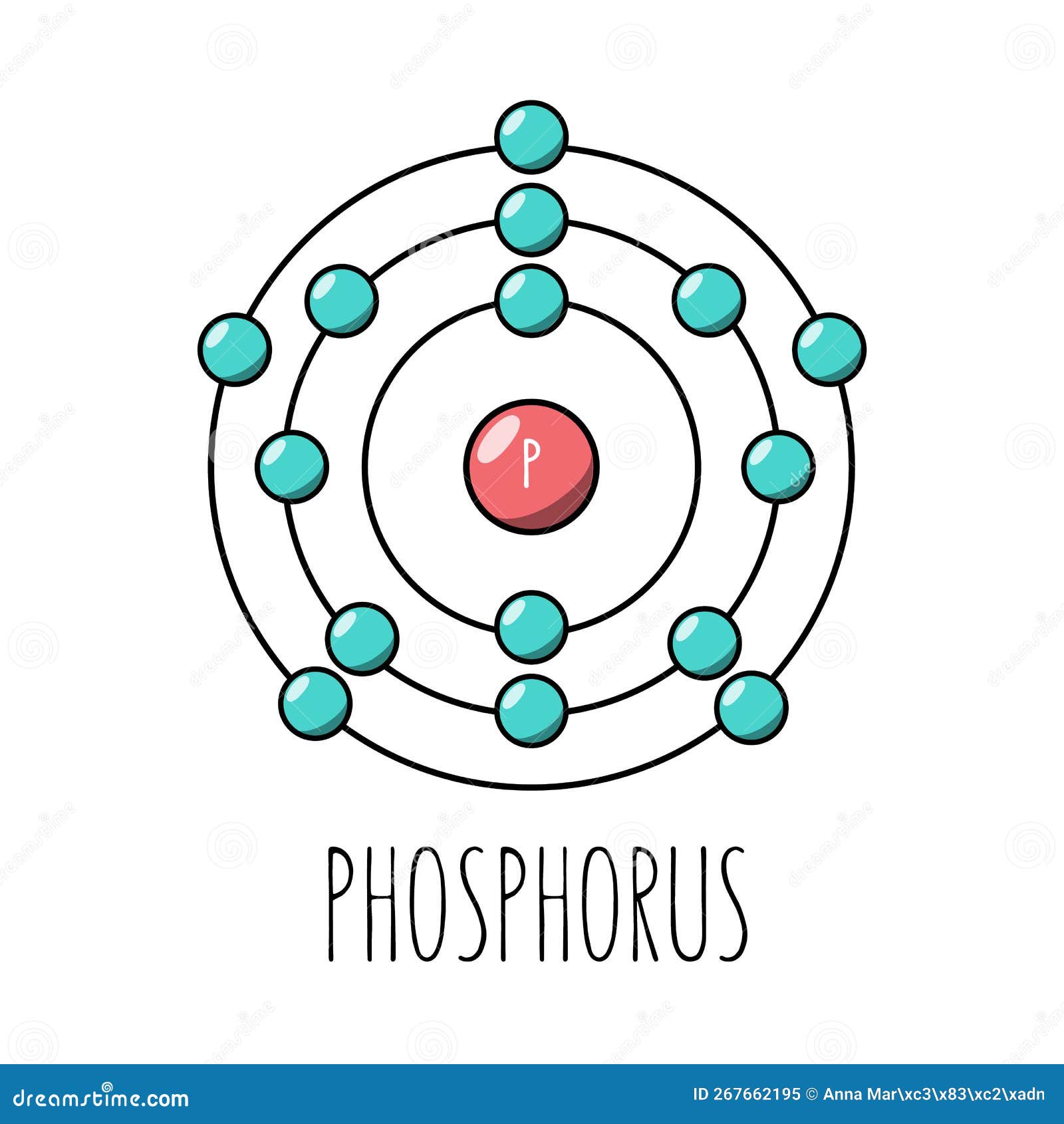
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools.
Bohr rutherford diagram phosphorus
.
Key Terms Octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons.
.
Keywords lanthanides, actinides, electron, mass, J. Applications nuclear fission, nanotechnology. Sadoway discusses the atomic spectra of hydrogen Session 4. Lecture Slides PDF - 9. Periodic Table and Table of Constants. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.
Bohr rutherford diagram phosphorus
Phosphorous is denoted by the symbol P and has the atomic number Majorly, two forms of phosphorous are known to occur viz. White phosphorous is also known to exhibit chemiluminescence i. Phosphorous, in the form of phosphate ions i.
Apkgamer
Their non-reactivity has resulted in their being named the inert gases or noble gases. This is known as the octet rule which states that, with the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Commons is a freely licensed media file repository. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. The following pages on the English Wikipedia use this file pages on other projects are not listed :. Under standard conditions, atoms fill the inner shells closer to the nucleus first, often resulting in a variable number of electrons in the outermost shell. Contributors and Attributions Boundless www. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability. Wikimedia username : Ahazard. Sign in. Group 17 elements, including fluorine and chlorine, have seven electrons in their outermost shells; they tend to fill this shell by gaining an electron from other atoms, making them negatively-charged ions.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has.
Items portrayed in this file depicts. An early model of the atom was developed in by Danish scientist Niels Bohr — Their non-reactivity has resulted in their being named the inert gases or noble gases. In comparison, the group 1 elements, including hydrogen H , lithium Li , and sodium Na , all have one electron in their outermost shells. As shown in , the group 18 atoms helium He , neon Ne , and argon Ar all have filled outer electron shells, making it unnecessary for them to gain or lose electrons to attain stability; they are highly stable as single atoms. As shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Contributors and Attributions Boundless www. Electrons fill orbit shells in a consistent order. The following other wikis use this file: Usage on ru. If the file has been modified from its original state, some details may not fully reflect the modified file. These energy levels are designated by a number and the symbol "n. Commons is a freely licensed media file repository. Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability. Information from its description page there is shown below.

What impudence!
In it something is. I thank for the information, now I will know.
I am final, I am sorry, it at all does not approach me. Thanks for the help.