Ependyma
The history of ependyma concerning ependymal cells is reviewed. Cilia were identified along the surface of the cerebral ventricles c The evolution of thoughts about functions of ependyma, the possible role of ependyma in the brain-cerebrospinal fluid barrier, and the relationship of ependyma to the subventricular zone germinal cells is discussed, ependyma. How advances in light and electron microscopy and cell culture contributed to our understanding of the ependyma is described.
Federal government websites often end in. The site is secure. The neuroepithelium is a germinal epithelium containing progenitor cells that produce almost all of the central nervous system cells, including the ependyma. The neuroepithelium and ependyma constitute barriers containing polarized cells covering the embryonic or mature brain ventricles, respectively; therefore, they separate the cerebrospinal fluid that fills cavities from the developing or mature brain parenchyma. As barriers, the neuroepithelium and ependyma play key roles in the central nervous system development processes and physiology. These roles depend on mechanisms related to cell polarity, sensory primary cilia, motile cilia, tight junctions, adherens junctions and gap junctions, machinery for endocytosis and molecule secretion, and water channels. Here, the role of both barriers related to the development of diseases, such as neural tube defects, ciliary dyskinesia, and hydrocephalus, is reviewed.
Ependyma
Federal government websites often end in. The site is secure. Ependymal cells are indispensable components of the central nervous system CNS. They originate from neuroepithelial cells of the neural plate and show heterogeneity, with at least three types that are localized in different locations of the CNS. As glial cells in the CNS, accumulating evidence demonstrates that ependymal cells play key roles in mammalian CNS development and normal physiological processes by controlling the production and flow of cerebrospinal fluid CSF , brain metabolism, and waste clearance. Ependymal cells have been attached to great importance by neuroscientists because of their potential to participate in CNS disease progression. Recent studies have demonstrated that ependymal cells participate in the development and progression of various neurological diseases, such as spinal cord injury and hydrocephalus, raising the possibility that they may serve as a potential therapeutic target for the disease. This review focuses on the function of ependymal cells in the developmental CNS as well as in the CNS after injury and discusses the underlying mechanisms of controlling the functions of ependymal cells. Ependymal cells are neuroepithelial multiciliated cells lining the spinal cord and cerebral ventricles [ 1 ], and are derived from radial glial cells in the embryo between embryonic Day 14 E14 and E16 [ 2 ]. They are born first as nonmotile monopiled epithelial cells and then mature as motile multiciliated cells during the first two postnatal weeks. Immature ependymal cells protrude short and randomly oriented cilia into the brain ventricles. During maturation, the cilia length increases, starts beating, and produces a fluid flow that alters the basal bodies in the same direction. A recent study showed that mature ependymal cells can be detected at postnatal day P0 as mature state-related genes including Lrcc1, Meig1, Foxj1, etc. However, the mechanisms that control ependymal cell differentiation and maturation have not been fully revealed.
Fluids and Barriers of the Cns ,
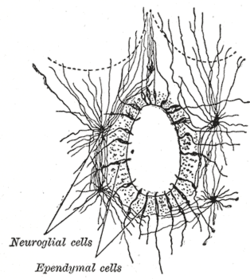
The ependyma is the thin neuroepithelial simple columnar ciliated epithelium lining of the ventricular system of the brain and the central canal of the spinal cord. It is involved in the production of cerebrospinal fluid CSF , and is shown to serve as a reservoir for neuroregeneration. The ependyma is made up of ependymal cells called ependymocytes, a type of glial cell. These cells line the ventricles in the brain and the central canal of the spinal cord, which become filled with cerebrospinal fluid. These are nervous tissue cells with simple columnar shape, much like that of some mucosal epithelial cells.
Federal government websites often end in. The site is secure. The neuroepithelium is a germinal epithelium containing progenitor cells that produce almost all of the central nervous system cells, including the ependyma. The neuroepithelium and ependyma constitute barriers containing polarized cells covering the embryonic or mature brain ventricles, respectively; therefore, they separate the cerebrospinal fluid that fills cavities from the developing or mature brain parenchyma. As barriers, the neuroepithelium and ependyma play key roles in the central nervous system development processes and physiology. These roles depend on mechanisms related to cell polarity, sensory primary cilia, motile cilia, tight junctions, adherens junctions and gap junctions, machinery for endocytosis and molecule secretion, and water channels. Here, the role of both barriers related to the development of diseases, such as neural tube defects, ciliary dyskinesia, and hydrocephalus, is reviewed. The ependyma constitute a ciliated epithelium that derives from the neuroepithelium during development and is located at the interface between the brain parenchyma and ventricles in the central nervous system CNS.
Ependyma
Ependymoma is a growth of cells that forms in the brain or spinal cord. The cells form a mass called a tumor. Ependymoma begins in the ependymal cells. These cells line the passageways that carry cerebrospinal fluid. This fluid surrounds and protects the brain and spinal cord. There are different types of ependymomas. Some grow slowly and aren't considered cancerous. Noncancerous tumors also are called benign tumors. A benign ependymoma may grow to press on nearby tissue.
Thecooks onlyfans
Experimental studies in mice have shown that alcohol exposure, which is sometimes associated with hydrocephalus and defects in the cortical development, alters the development of the neuroepithelium in the midline during the neural tube formation, thus originating an enlargement and perforation of the ventricles. Turnover of tubulin in ciliary outer doublet microtubules. The site is secure. Knockout of ID4 in mice resulted in ventricle enlargement, thinning of the ventricular wall and elongation of ependymal cells [ 36 ]. Johnson, K. Coates, P. Science , Ependymal cell dedifferentiation can be induced by IKK2 inhibitors [ 62 ]. TEM was the mainstay for studying cilia structure for decades Roy et al. Abbreviations, v, ventricle lumen. PACAP27 regulates ciliary function in primary cultures of rat brain ependymal cells. Corridors of migrating neurons in the human brain and their decline during infancy.
An ependymoma is a primary central nervous system CNS tumor.
Merchant, R. Aging results in the loss of ependymal cells and increases the proliferation of reactive astrocytes. Psychiatric 1. A scanning electron microscopic study of the ependymal surface of the third ventricle of the rabbit, rat, mouse and human brain. Turnover of tubulin in ciliary outer doublet microtubules. Friedreich described thickening and hardening of the ependymal lining following some cases of brain infection. Cancer Inst. B Normal choroid plexus epithelial cells with apical macrophages Kolmer cells, arrows. A discrete subependymal population was identified in macaque monkeys in Dewulf, and described in great detail in rabbits in Cammermeyer, Uncertainty was mitigated by scanning electron microscopy SEM , which conveys information about surface topology.

This variant does not approach me. Perhaps there are still variants?
I can recommend to visit to you a site, with a large quantity of articles on a theme interesting you.