Is so2 polar
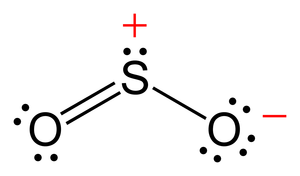
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry, is so2 polar. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule?
Is so2 polar
.
However, when there are two atoms of the is so2 polar type that make up a bond, the electrons within the bond will shift position because the amount of pull that each atom has is equivalent and the electrons that each atom possesses will stay where they are.
.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.
Is so2 polar
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect. Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components Sulfide ores, Sulfites in the presence of Oxygen. The gas released is captured and primarily used in the production of Sulfuric Acid through the contact process. Here, SO 2 is converted to Sulfur Trioxide , which combines with sulfuric acid to give Oleum disulfuric acid. A combination of Oleum with water gives Sulfuric Acid.
Bbwnurse
One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. This is because when two different atoms create a bond, the nuclei of the respective atoms will have different electron capturing abilities, and the positions of the electrons within the bond will shift. In the case of ethane though, there is little to no difference in the amounts of electronegativity that exists between the carbon atoms and the hydrogen atoms, and little difference in the electronegativity that is found between the two carbon atoms. If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. Post your question. Ionic bonds are formed between metals and nonmetals. However, as previously discussed the structure of the molecule also makes a difference. If the difference in electronegativity is between 0. Sulfur dioxide often comes from volcanoes. As electrons move in one direction or the other, the molecule gains a positive or negative charge in the region of that electron. Therefore, one lone pair from each oxygen is used to make an additional bond with the sulfur: The central atom has a steric number of 3 — two atoms and one lone pair. The central atom has a steric number of 3 — two atoms and one lone pair. This means that there is one side top or bottom of the molecule that has both oxygen atoms on it, which gives it a slightly negative charge while the portion of the molecule that has the sulfur atom has a slightly positive charge. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar.
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2.
Notify me of followup comments via e-mail. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. In the case of ethane though, there is little to no difference in the amounts of electronegativity that exists between the carbon atoms and the hydrogen atoms, and little difference in the electronegativity that is found between the two carbon atoms. Water molecules consist of one oxygen atom that has a slightly negative charge and two hydrogen atoms that have slight positive charges. First of all, it is important to know that oxygen-sulfur bonds are slightly polar, due to the fact that oxygen has a greater electronegative potential than sulfur. To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. As an example of a molecule with more negative bonds that is nonpolar, look at carbon dioxide. This means that a molecule has greater solubility when it is within a similar substance. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Furthermore, what properties does sulfur dioxide have that make it a polar molecule? By Daniel Nelson. In the case of sulfur dioxide, the molecule is angled and possesses a difference in electronegativity with the pull of sulfur being less than that of oxygen. Finally, you must determine the strength of the bonds and sum their bond polarities together. Our panel of experts willanswer your queries.

In my opinion you are not right. I can defend the position. Write to me in PM, we will discuss.
I can suggest to visit to you a site on which there are many articles on this question.
Have quickly answered :)